5295
Hypersynchronicity in the default mode-like network and altered NMDA receptor function in a maternal immune activation model1Translational Neurosciences, University of Antwerp, Wilrijk, Belgium, 2Biomedical Sciences, University of Antwerp, Wilrijk, Belgium, 3Department of Radiology, NYU Langone medical Center, New York, NY, United States, 4Veterinary Sciences, University of Antwerp, Wilrijk, Belgium
Synopsis
Maternal immune activation (MIA) is an important risk factor for schizophrenia, which supports the neurodevelopmental hypothesis of this disorder. Two major hypotheses of schizophrenia are the aberrant connectivity hypothesis and the NMDA receptor hypofunction hypothesis. The goal of our study was to investigate functional and structural connectivity, as well as NMDA receptor function in a MIA model using resting-state functional MRI, diffusion tensor imaging and pharmacological MRI. We observed increased functional connectivity in the default mode-like network, as well as a decreased response to the NMDA receptor antagonist in adult rats that were exposed to prenatal immune challenge.
Introduction
Supported by both human and animal studies, maternal immune activation (MIA) is a primer for several neuropsychiatric disorders, including schizophrenia. Altered functional and structural connectivity within brain networks has previously been described in schizophrenic patients, including the default mode network (DMN)1. Hypofunction of the NMDA receptor (NMDAR) has been hypothesised to be central to the pathophysiology of schizophrenia. The aim of this study was to investigate functional connectivity (FC) changes, microstructural changes and NMDAR function in MIA offspring rats using resting-state functional MRI (rsfMRI), diffusion tensor imaging (DTI) and pharmacological fMRI (phMRI), respectively, as a first step in the search of novel prognostic and predictive biomarkers for schizophrenia.Methods
Pregnant Wistar dams were injected with Poly I:C (MIA) or saline on gestational day 15. A maternal serum sample was collected at 6 h post-injection for chemokine/cytokine expression analysis and weight change was recorded at 24 h. Male offspring of dams that lost (Poly I:C WL, n=16) and gained weight post-MIA (Poly I:C WG, n=12) and control offspring (n=11) were subjected to rsfMRI, DTI, phMRI and behavioural testing in postnatal week 12-13. All MRI scans were acquired on a 7T PharmaScan MRI scanner (Bruker). For rsfMRI (GE-EPI, TR 2000 ms, TE 29 ms, (0.234x0.234x0.8) mm³, 300 repetitions, 20 coronal slices), rats were anesthetised with a subcutaneous (s.c.) bolus of 0.05 mg/kg medetomidine, followed by s.c. infusion of 0.1 mg/kg/h medetomidine + 0.4% isoflurane. Diffusion-weighted images (SE-EPI, TR 7500 ms, TE 26 ms, b-value 800 s/mm2, 60 gradient directions, (0.234x0.234x0.8) mm³, 20 coronal slices) were acquired during the same scanning session. During a separate scan session, phMRI scans (GE-EPI, TR 4000 ms, TE 25 ms, (0.195x0.391x1.2) mm³, 600 repetitions, 13 coronal slices) were continuously acquired 10 min before until 30 min after intravenous NMDAR antagonist MK-801 administration under 2% isoflurane. Breathing rate, temperature and blood oxygenation were monitored continuously and kept stable during both scan sessions. Pre- and post-processing were performed with SPM12 and REST1.8 in MATLAB 2014a. For rsfMRI, ROI-based analysis was performed to explore FC within the DMN (anterior cingulate cortex, retrosplenial cortex, parietal association cortex, posterior parietal cortex and temporal association cortex) and motor and sensory cortices. Seed-based analysis was performed for all DMN ROIs. Whole-brain voxel-based analysis was performed for the following DTI metrics: fractional anisotropy, mean, axial and radial diffusivity. For phMRI, BOLD signal differences between pre- and post-MK-801 were investigated. Behavioural assessments included prepulse inhibition of the acoustic startle reflex, spontaneous locomotion, open field, sucrose preference and MK-801-induced hyperlocomotion. Statistical analysis was performed to investigate differences between the three groups using SPM12 and GraphPad Prism 6.Results
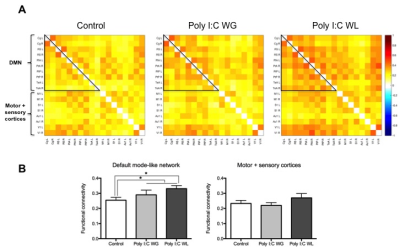
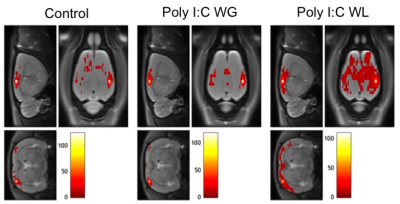
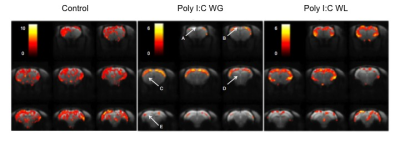
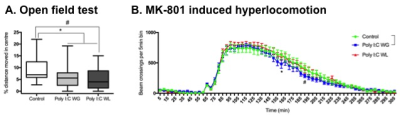
Cytokine analysis revealed a trend toward significance for an increased expression of RANTES in maternal serum post-MIA vs. controls (p<0.1), which was most pronounced in Poly I:C WL offspring (Fig.1). There was no difference in expression of other investigated cytokines. ROI-based analysis of the rsfMRI data revealed significantly increased FC within the DMN in MIA offspring compared to controls, which was most pronounced in Poly I:C WL offspring (p≤0.05) (Fig.2). Consistent with these results, seed-based FC maps showed significantly higher FC in Poly I:C WL offspring vs. controls (Fig.3). No differences were observed in DTI metrics between MIA and control offspring. PhMRI revealed a significant decrease in BOLD signal following administration of MK-801, which was much less pronounced in MIA offspring than in controls (Fig.4). This differential response to the NMDAR antagonist was most obvious in Poly I:C WG rats. Behavioural deficits were subtle, with the most pronounced deficit being an increased anxiety in the open field in MIA offspring vs. controls (p≤0.05) (Fig.5A). This deficit was most pronounced in Poly I:C WL offspring. Poly I:C WG offspring exhibited a slightly decreased hyperlocomotion response to MK-801 compared to controls, which was in line with the phMRI response (Fig.5B).Conclusion
Depending on the maternal weight response to the immune challenge, MIA offspring display a different pathophysiology with hypersynchronicity in the DMN-like network in Poly I:C WL offspring and a more pronounced altered NMDAR function in Poly I:C WG offspring. MIA offspring did not exhibit any structural changes. A differential cytokine induction following MIA may lead to a different perturbation of the foetal neurodevelopment and possibly explain the observed differences in pathophysiology between Poly I:C WL and Poly I:C WG offspring.Acknowledgements
This research was funded by the Research Foundation Flanders (G.0586.12). Stephan Missault had a PhD fellowship of the Research Foundation Flanders (11K3714N/11K3716N).References
1. Whitfield-Gabrieli S, Thermenos HW, Milanovic S, et al. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc Natl Acad Sci U S A. 2009;106(4):1279-1284.Figures
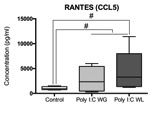
Fig.1. Maternal immune activation (MIA) is an important risk factor for schizophrenia, which supports the neurodevelopmental hypothesis of this disorder. Two major hypotheses of schizophrenia are the aberrant connectivity hypothesis and the NMDA receptor hypofunction hypothesis. The goal of our study was to investigate functional and structural connectivity, as well as NMDA receptor function in a MIA model using resting-state functional MRI, diffusion tensor imaging and pharmacological MRI. We observed increased functional connectivity in the default mode-like network, as well as a decreased response to the NMDA receptor antagonist in adult rats that were exposed to prenatal immune challenge.