5289
Accelerated Isotropic Sub-Millimeter Whole-Brain Susceptibility Imaging at 3T: Application to Multiple Sclerosis1Siemens Medical Solutions USA, Inc, Baltimore, MD, United States, 2Radiology and Imaging Sciences, University Of Utah, Salt Lake City, UT, United States, 3Section of Functional Imaging Methods, Laboratory of Brain and Cognition, National Institute of Mental Health, National Institutes of Health, Bethesda, MD, United States, 4Siemens Healthcare, Zurich, Switzerland, 5Siemens Medical Solutions USA, Inc, Boston, MA, United States, 6Translational Neuroradiology Section, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, United States
Synopsis
High-resolution susceptibility-weighted MRI has recently gained attention as a novel imaging biomarker in multiple sclerosis (MS) such as the ‘central vein sign’ and ‘phase rims’. This preliminary study demonstrates the feasibility of an accelerated volumetric (3D) segmented echo-planar-imaging (EPI) sequence, which allows acquiring whole brain images at 0.65 mm isotropic resolution in approximately 3 minutes. Both magnitude (T2*-weighted) and phase (QSM) information were interpretable and displayed characteristic features of MS lesions. This accelerated 3D EPI anatomical acquisition shows potential to open the door to routine high-resolution susceptibility-weighted imaging to better support diagnosis and therapy monitoring in MS patients.
Introduction
High-spatial-resolution susceptibility-weighted imaging of the brain anatomy has recently gained a lot of attention for investigating novel biomarkers in multiple sclerosis (MS) such as the ‘central vein sign’ which may aid with MS diagnosis1 and ‘phase rims’ which may predict the outcome of new MS lesions2. To allow rapid susceptibility-based imaging of the entire brain at submillimeter resolution, we have developed a volumetric (3D) segmented echo-planar-imaging (EPI) sequence3. In this study, we aimed at demonstrating the feasibility of an accelerated version of our prototype sequence using parallel imaging which allows acquiring whole brain images at 0.65 mm isotropic resolution in approximately 3 minutes.Methods
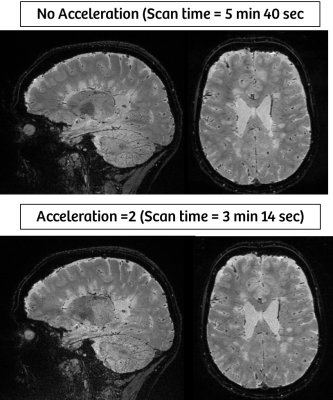
Generalized Auto-calibrating Partially Parallel Acquisition (GRAPPA) was implemented on a previously introduced 3D EPI prototype sequence3. The accelerated prototype sequence was tested on a 3T scanner (MAGNETOM Skyra, Siemens Healthcare, Erlangen, Germany) equipped with a 32-channel RF-receive head coil. A high-resolution (0.65 mm isotropic) protocol was setup for imaging the small parenchymal veins running through MS white matter lesions. Main parameters were as follow: FOV = 250 (SI) x 203 (AP) x 166 (RL) mm3, matrix = 384 x 312 x 256, TR = 64 ms, TE = 35 ms, FA = 100, EPI factor = 15, R = 2, scan time = 3 min 14 sec. Three subjects with MS were scanned to assess the feasibility of this clinically-compatible protocol for detecting central veins and phase rims. The same sequence was repeated without acceleration (no GRAPPA, scan time = 5 min 40 sec) to compare image quality. Both magnitude (T2*-weighted) and phase images were collected. Phase images were further post-processed for quantitative susceptibility mapping (QSM)4.Results
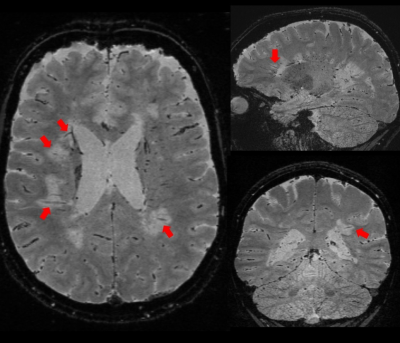
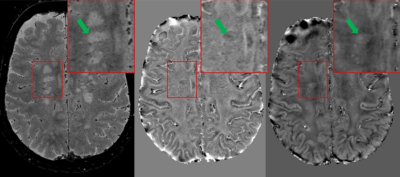
High-resolution T2*-weighted images (Figure 1) of the whole brain were collected in ~ 3 minutes using the 3D EPI prototype sequence with parallel imaging (GRAPPA). White matter lesions and small medullary veins (red arrows) were readily detected on T2* contrast. Some of the white matter lesions displayed a paramagnetic rim on the corresponding phase and QSM images (Figure 2, green arrows). When compared with the non-accelerated images, the loss in SNR due to the total acceleration factor of 2 only minimally affected the overall image quality (Figure 3).Discussion
This preliminary study demonstrates the favorable initial experience with a two-fold accelerated version of our prototype sequence which allows acquisition of T2*-weighted whole brain images at 0.65 mm isotropic resolution in approximately 3 minutes. Both magnitude (T2*-weighted) and phase (QSM) information were interpretable and displayed characteristic features of MS lesions which could help with diagnosis and monitoring of patients. Reaching scan times in a few minutes range to acquire whole brain high resolution T2*-weighted images is expected to ease the way into clinical routine protocols to investigate the potential of the new central vein imaging biomarker in MS. Currently testing is underway to explore higher acceleration factors (e.g. R = 2 x 2) as well as CAIPIRINHA acceleration schemes which is known to improve g-factor and thereby provide better SNR at high acceleration factors5 using 32- and 64-channel receive arrays.Conclusion
In this pilot study, we demonstrated the feasibility of a two-fold accelerated high resolution 3D EPI prototype sequence which acquires whole-brain images at 0.65 mm isotropic resolution in approximately 3 minutes. This enables the routine acquisition of high-resolution isotropic MR susceptibility brain imaging in patients with MS.Acknowledgements
No acknowledgement found.References
1. Sati P, Oh J, Constable RT, et al. The central vein sign and its clinical evaluation for the diagnosis of multiple sclerosis: a consensus statement from the North American Imaging in Multiple Sclerosis Cooperative. Nat Rev Neurol. 2016 Dec;12(12):714-722.
2. Absinta M, Sati P, Schindler M, et al. Persistent 7-tesla phase rim predicts poor outcome in new multiple sclerosis patient lesions. J Clin Invest. 2016 Jul 1;126(7):2597-609.
3. Sati P, Patil S, Inati S et al. Rapid MR Susceptibility Imaging of the Brain Using Segmented 3D Echo-Planar Imaging (3D EPI) and its Clinical Applications. MAGNETOM Flash (68) 2/2017.
4. Langkammer C, Bredies K, Poser BA, et al. Fast Quantitative Susceptibility Mapping using 3D EPI and Total Generalized Variation. Neuroimage. 2015 May 1;111:622-30.
5. Breuer FA, Blaimer M, Mueller MF, Seiberlich N, Heidemann RM, Griswold MA, Jakob PM. Controlled aliasing in volumetric parallel imaging (2D CAIPIRINHA). Magn. Reson. Med. 2006;55:549–556. doi: 10.1002/mrm.20787
Figures