5283
Quantification of white matter tract integrity in primary-progressive multiple sclerosis1Icahn School of Medicine, New York, NY, United States, 2University of Genoa, Genoa, Italy
Synopsis
Diffuse white matter (WM) injury is prominent in primary progressive multiple sclerosis (PPMS). Diffusion Kurtosis Imaging (DKI) allows the quantification of non-Gaussian water diffusion, offering the possibility of more detailed characterization of WM damage, in comparison with that provided by diffusion tensor imaging metrics. Here we present application of DKI metrics in PPMS using a Tract-Based Spatial Statistics approach. We observed a diffuse WM microstructural damage, manifested as axonal water fraction, mean kurtosis and fractional anisotropy decrease. In line with histopathological studies, our results suggest the prevalence of axonal damage over demyelination in progressive MS.
Purpose
Diffuse white matter (WM) injury is prominent in primary progressive multiple sclerosis (PPMS) pathology and is a potential biomarker of disease progression associated with increase of disability from disease onset. Diffusion Kurtosis Imaging (DKI) allows the quantification of non-Gaussian water diffusion [1], offering the possibility of more detailed characterization of WM damage, in comparison with that provided by traditional diffusion tensor imaging (DTI) metrics, such as fractional anisotropy (FA) and mean diffusivity (MD). In this work we present for the first time a validation of DKI metrics in PPMS using a Tract-Based Spatial Statistics (TBSS) approachMethods
26 PPMS patients (14F, mean age 50.92±10.30 years, median Expanded Disability Status Scale-EDSS 4.0, EDSS range 1.5-6.0) and 20 healthy controls (HC) (11F, mean age 51.05±9.80 years) were enrolled for this study. DKI single-shot EPI images where acquired on a 3T Achieva scanner, Philips with a voxel size of 2×2×2 mm3, 30 directions for each b-values=1000,2000s/mm2 and one b=0s/mm2. Diffusion and Kurtosis tensors were calculated using Diffusional Kurtosis Estimator (DKE) software [2], to obtain FA, MD and mean kurtosis (MK) spatial maps. A 2-compartment biophysical model of WM fiber bundles was used to derive spatial maps of axonal water fraction (AWF), intra-axonal diffusivity (Daxon), extra-axonal axial diffusivity (De||), extra-axonal radial diffusivity (De┴) and tortuosity of the extra-axonal space spatial maps. Voxelwise statistical analysis of the DTI metrics was carried out using TBSS [3], part of FSL. Non-parametric permutation inference using randomise was used for voxelwise statistics (5000 permutations, TFCE, p=0.05).Results and Discussion
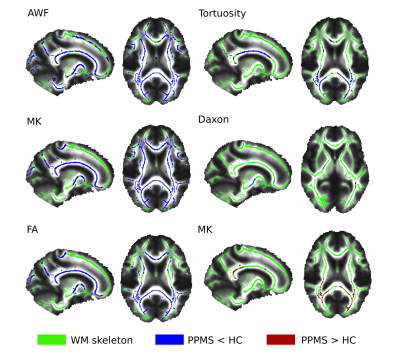
The comparison of PPMS and HC disclosed the presence of a widespread decrease in FA, MK and AWF in body of the corpus callosum, right anterior thalamic radiation, inferior longitudinal fasciculus, inferior fronto-occipital fasciculus, posterior thalamic radiation and optic radiation. Similar findings were identified for tortuosity, mainly right lateralized, with decrease in body and splenium of the corpus callosum, posterior thalamic radiation, right anterior limb of internal capsule and bilateral posterior internal capsule. MD was increased in the splenium of the corpus callosum, posterior thalamic radiation, optic radiation and inferior fronto-occipital fasciculus. Significant decrease in Daxon was detected in right anterior and posterior limbs of the internal capsule, right cerebellar peduncle, right anterior corona radiata, and left posterior thalamic radiation. No significant changes were detected in De|| and De┴.Conclusion
Damage of WM microstructure in PPMS patients was observed in most WM tracts, mainly manifested as AWF, MK and FA decrease. Our results suggest, in line with histopathological studies [4], the prevalence of diffuse chronic axonal damage over demyelination in the progressive phenotype. AWF and Mean Kurtosis appear to be the most sensitive metrics to tissue damage while De|| and De┴ seem to be the least sensitive.Acknowledgements
NMSS RG 5120A3/1References
[1] Lu H, Jensen JH, Ramani A, Helpern JA. Three-dimensional characterization of non-Gaussian water diffusion in humans using diffusion kurtosis imaging. NMR Biomed. 2006 Apr;19(2):236-47.
[2] Tabesh A, Jensen JH, Ardekani BA, Helpern JA. Estimation of tensors and tensor-derived measures in diffusional kurtosis imaging. Magn Reson Med. 2011 Mar;65(3):823-36.
[3] Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, and Behrens TEJ. Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. 2006 Jul 15;31(4):1487-505.
[4] Lassmann H, van Horssen J, Mahad D. Progressive multiple sclerosis: pathology and pathogenesis. Nat Rev Neurol. 2012 Nov 5;8(11):647-56
Figures