5282
Altered Hippocampal GABA and Glutamate Levels and Uncoupling from Functional Connectivity in Multiple Sclerosis1Shandong Medical Imaging Research Institute, Jinan, China, 2Southwest Hospital, Third Military Medical University, chongqing, China, 3Philips Healthcare, shanghai, China
Synopsis
This study offers a novel combination of methods investigating the complex relationships among excitatory/inhibitory neurotransmitters, brain connectivity and cognitive function in health and disease states. Modulation of Glu and GABA neurotransmission may enable the development of new therapeutic strategies for the early stages of MS.
Purpose
There is growing evidence for dysfunctional glutamatergic excitation and/or gamma-aminobutyric acid (GABA)ergic inhibition in patients with multiple sclerosis (MS)1, 2. Cognitive impairment may occur during the early stages of MS and hippocampal abnormalities have been suggested as biomarkers. However, researchers have not clearly determined whether changes in hippocampal GABA and glutamate (Glu) levels are associated with cognitive impairment and aberrant neural activity in patients with MS.Material and Methods
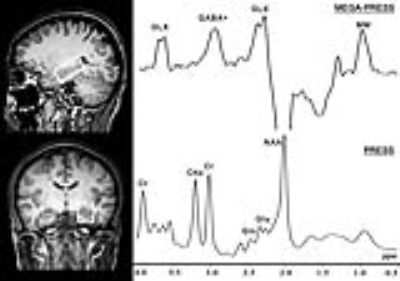
Twenty-nine patients with RRMS (8 males, mean age: 36.38 ± 9.86 years) and twenty-nine healthy controls (10 males, mean age: 37.38 ± 10.46 years) were recruited in this study. All subjects were scanned with a 3.0 T scanner (Philips Achieva). The VOI with a size of 4 × 2 × 2 cm3 was centered on the left hippocampus (Fig. 1). The GABA level was measured using the MEGA-PRESS sequence and the Glu level was obtained from the same VOI using a PRESS sequence. The resting-state fMRI data were acquired using an echo-planar gradient-echo pulse sequence. To extract the FC strengths for each participant, we performed seed-based connectivity analyses, using the clusters showing significant between-group differences in the hippocampal FC analysis as the seeds. The subjects’ neuropsychological statuses were tested using the Auditory Verbal Learning Test (AVLT) for verbal learning and memory, the Rey-Osterrieth Complex Figure Test (ROCF) for visuospatial memory.Results
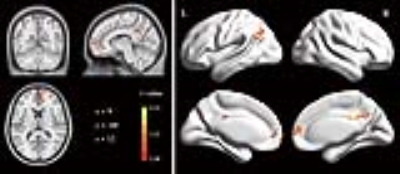
Compared with the HCs, patients with MS showed significantly lower GABA+ levels in the left hippocampus, which were reduced by 11.59% (2.33 ± 0.43 iu vs. 2.06 ± 0.46 iu, p = 0.03, Fig. 2). In addition, the difference in Glu levels also reached significance (11.92 ± 1.55 iu vs. 11.02 ± 1.52 iu, p = 0.03, Fig. 2). No significant difference in the Glu/GABA+ ratio (5.31 ± 1.27 vs. 5.57 ± 1.37, p = 0.50, Fig. 2) was observed between groups. Compared with the HCs, MS patients exhibited lower connectivity with the left hippocampus in three clusters, including the bilateral medial prefrontal cortices (MPFC), left angular gyrus (AG) and bilateral PCC (Fig. 3). In the HC group (Fig. 4), partial correlation analyses revealed that the GABA+ in the left hippocampus was positively correlated with the hippocampal FC with the AG (ρ = 0.35, p = 0.047) and the PCC (ρ = 0.53, p = 0.005), whereas the Glu/GABA+ ratio was negatively correlated with the hippocampus-PCC connectivity (ρ = -0.40, p = 0.04). GABA+ levels also tended to be associated with the hippocampus-MPFC connectivity (ρ = 0.43, p = 0.09). In the MS group, correlations were not observed between neurotransmitter levels and FC strengths (p > 0.05, uncorrected; Fig. 4). In the MS group, the regression analyses found that the best predictors for AVLT scores (R2 = 0.36, p = 0.003) were the GABA+ levels (β = 0.53, p = 0.002) and age (β = -0.33, p = 0.049), whereas age (β = -0.54, p = 0.001) and Glu levels (β = 0.38, p = 0.02) were used to explain the total variability in ROCF scores (R2 = 0.41, p = 0.001).Discussion
This study is the first to show that patients with RRMS exhibit both lower GABA+ and Glu levels in the hippocampus, which may reflect dysfunctional glutamatergic and GABAergic systems. The abnormalities in the excitatory and inhibitory neurotransmitter systems may be attributed to neurodegeneration and demyelination, which are prominent features observed in the hippocampus of patients with MS 3. Our analysis in the HC group closely links the hippocampal FC strengths with the GABA+ levels and Glu/GABA+ ratios, providing a more precise delineation of the biochemical underpinnings of macro-scale brain connectivity. In contrast, the uncoupling of hippocampal FC strengths and GABA+ levels or the Glu/GABA+ ratios in patients with MS suggest that altered neurotransmitter control of synchronized BOLD signal fluctuations between the hippocampus and its input or target areas.Conclusion
By combining J-difference-edited MRS and resting-state fMRI, we provided evidence that hippocampal GABA+ and Glu levels, as well as strengths of FC within brain regions belonging to the DMN, were abnormal in patients with MS. In addition, the reduced verbal memory and visuospatial memory observed in patients with MS correlated with decreased levels of GABA+ and of Glu, respectively. Finally, GABA+ levels and Glu/GABA+ ratios were associated with FC strengths in HCs but not in patients with MS. This study offers a novel combination of methods investigating the complex relationships among excitatory/inhibitory neurotransmitters, brain connectivity and cognitive function in health and disease states. Modulation of Glu and GABA neurotransmission may enable the development of new therapeutic strategies for the early stages of MS.Acknowledgements
This work was supported by the Shandong Provincial Natural Science Foundation of China (no. BS2015YY003); National Natural Science Foundation of China for Young Scholars (no. 81601479); and Shandong Provincial Key Research and Development Plan of China (no. 2016GSF201090).References
[1] Cawley N, Solanky B S, Muhlert N, Tur C, Edden R A, Wheeler-Kingshott C A, Miller D H, Thompson A J, Ciccarelli O. Reduced gamma-aminobutyric acid concentration is associated with physical disability in progressive multiple sclerosis [J]. Brain, 2015, 138(Pt 9): 2584-2595.
[2] Nantes J C, Proulx S, Zhong J, Holmes S A, Narayanan S, Brown R A, Hoge R D, Koski L. GABA and glutamate levels correlate with MTR and clinical disability: Insights from multiple sclerosis [J]. Neuroimage, 2017
[3] Papadopoulos D, Dukes S, Patel R, Nicholas R, Vora A, Reynolds R. Substantial archaeocortical atrophy and neuronal loss in multiple sclerosis [J]. Brain Pathol, 2009, 19(2): 238-253.
Figures