5278
1H MRS study of glutamate excitotoxicity in hypothalamus of early multiple sclerosis patientsPetra Hnilicová1, Ema Kantorová2, Hubert Poláček 3, Štefan Sivák2, Michal Bittšanský1, Kamil Zeleňák4, Egon Kurča2, and Dušan Dobrota 5
1Division of Neurosciences, Biomedical Center Martin, Jessenius Faculty of Medicine in Martin, Comenius University in Bratislava, Martin, Slovakia, 2Clinic of Neurology, Jessenius Faculty of Medicine in Martin, Comenius University in Bratislava, Martin, Slovakia, 3Clinic of Nuclear Medicine, Jessenius Faculty of Medicine in Martin, Comenius University in Bratislava, Martin, Slovakia, 4Clinic of Radiology, Jessenius Faculty of Medicine in Martin, Comenius University in Bratislava, Martin, Slovakia, 5Department of Medical Biochemistry, Jessenius Faculty of Medicine in Martin, Comenius University in Bratislava, Martin, Slovakia
Synopsis
Multiple sclerosis (MS) is considered as an autoimmune disease with expanding axonal and neuronal degeneration in the spinal cord or cerebral cortex during the acute MS phase. The hypothalamus (HYP) is often overlooked yet controls important homeostatic functions. This 1H MRS study performed on 1.5 T MR scanner using 3D CSI with 10×10×12.5 mm3 voxels was focused to altered HYP metabolism in early MS. Considering our results, increased Glx ratios with reduced mIns and tNAA ratios in HYP suggested glutamate excitotoxicity associated with glial activity and neuronal damage. This indicated that HYP plays an important role in early disease evolution.
INTRODUCTION
Multiple Sclerosis (MS) is believed to result from a cell-mediated autoimmune response that initiates myelin loss, axonal as well as neuronal damage and in the acute phase of disease evolution leads to the progressive neurological dysfunction1,2. Signs and symptoms of MS are usually attributed to demyelinating lesions across the white matter (WM)2,3. However, recent research has shown that the neurodegenerative process is rather associated to extremely complex neuro-axonal pathologies4,5. It is also proposed that deep gray matter (DGM) is injured initially with high extension in the early stage of MS2,6. One of the most essential DGM regions affected by MS is hypothalamus (HYP), which is involved in many necessary body processes including behavioral, autonomic and endocrine functions, such as metabolism, development, and some fundamental systemic regulations (e.g. circadian rhythms, blood pressure, thermoregulation)1,5,7.METHODS
To the study were enrolled 31 adult patients (27±6 years of age, 12 men/19 women) in early stage of MS and 31 age- and gender- matched healthy volunteers. Measurements were performed on a 1.5 T whole body MR scanner Magnetom Symphony using the 8-channel head coil for signal reception (Siemens, Erlangen, Germany). The study protocol contained T2-weighted and FLAIR MRI sequences, anatomical T1-weighted MP-RAGE scanning and B0 phase mapping enabling to reach the eligible volume shimming (Figure 1), directly in the hypothalamic area outside of any B0 distortions visible on the B0 map. For 1H MRSI data acquisition was used 3D CSI (PRESS, TR/TE=1500/30 ms, NA=4, FOV=100×100×100 mm3, VOI=50×60×35 mm3) with 10×10×12.5 mm3 nominal voxel size (10×10×8 CSI grid interpolated to 16×16×8 matrix) and total acquisition time of 7min 42s. In post-processing (Figure 2), the two required CSI voxels were placed in the right and left hypothalamus using jSIPRO interface (Jiru & Skoch, Czech Republic). Spectra were evaluated in LCModel software (S. Provencher, Canada) for obtaining integrals of N-acetylaspartate with N-acetyl-aspartyl-glutamate (tNAA), creatine containing compounds (tCr), glutamate with glutamine (Glx), and myo-Inositol (mIns). All MR spectra fulfilled defined spectral quality criteria, i.e. spectral SNR ≥ 10 and the FWHM of tNAA peak ≤ 10 Hz. Finally, differences in metabolite ratios (tNAA/tCr, Glx/tCr, Glx/tNAA, mIns/tCr, and mIns/tNAA) between patients and controls were evaluated using paired-samples two-sided t-test in SPSS software (Chicago, USA). The p-value <0.05 was considered significant.RESULTS
In HYP of early MS patients compared to the healthy controls were found highly increased Glx metabolite ratios in contrast to significantly lower tNAA/tCr (Table). Although, no group differences were observed in mIns/tCr, the mIns/tNAA ratio was significantly elevated for the MS group.DISCUSSION
Increased Glx ratios in HYP of MS patients may reflect obvious glutamate excitoxicity which seems to be the most pronounced pathology affecting the neurodegenerative processes in early stage of disease4,5,7. It is linked with decline of tNAA ratios indicating axial and neuronal dysfunction and/or loss2,6. Excessive glutamate concentrations may also affect neuroglial homeostasis and cause microglial activation, both associated with increased mIns ratios2,5. Since tCr and mIns are considered to be glial and tNAA neuronal markers2,3,4 the results postulated slightly initiation of gliosis, whereas neurodegeneration occurs very progressive. The involvement of HYP to MS pathology can explain physiologic homeostasis instability, physical disability, and cognitive impairment of patients, even in early MS stage1,7.CONCLUSION
Considering our results, during the early MS progression, hypothalamic tissue shown increasing glutamate excitotoxicity causing marked neuronal loss and slow glial activation. Therefore, there is a potential for Glx ratios to be used as a marker of the progression and severity of MS, even in patients at early stage of diseases. Furthermore, it is shown up that the underlying pathological processes in DGM during MS are different from WM damage and it is necessary to continue in further investigations.Acknowledgements
This study was supported by the projects: “Biomedical Center Martin, ITMS: 26220220187“, APVV-14-0088, and VEGA 1/0287/16, co-funded from EU sources and European Social Fund.References
- Messina S, Patti F. Gray Matters in Multiple Sclerosis: Cognitive Impairment and Structural MRI. Mult Scler Int. 2014.
- Sajja BR, Wolinsky JS, Narayana PA. Proton Magnetic Resonance Spectroscopy in Multiple Sclerosis. Nuroimaging Clin N Am. 2009; 19(1): 45-58.
- Ruiz-Peña JL, Piñero P, Sellers G, et al. Magnetic resonance spectroscopy of normal appearing white matter in early relapsing-remitting multiple sclerosis: correlations between disability and spectroscopy. BMC Neurology. 2004; 4:8.
- Macrez R, Stys PK, Vivien D, et al. Mechanisms of glutamate toxicity in multiple sclerosis: biomarker and therapeutic opportunities. Lancet Neurol. 2016;15(10):1089-102.
- Deckx N, Lee WP, Berneman ZN, Cools N. Neuroendocrine Immunoregulation in Multiple Sclerosis. Clin Dev Immunol. 2013.
- Hulst HE, Geurts JJG. Gray matter imaging in multiple sclerosis: what have we learned? BMC Neurology. 2011; 11:153.
- Zivadinov R, Pirko I. Advances in understanding gray matter pathology in multiple sclerosis: Are we ready to redefine disease pathogenesis? BMC Neurol. 2012; 12: 9.
Figures
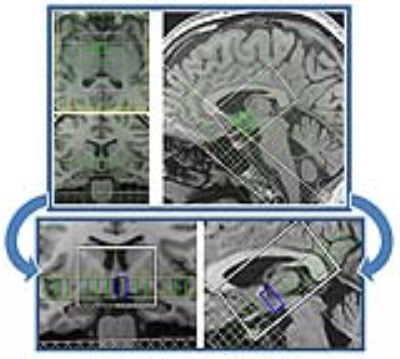
1H MRS measurement in hypothalamus (blue rectangle) performed at 1.5 T with
precise placement of eligible shimming volume (green rectangle). T1-weighted MP-RAGE images display 10×10×8 CSI grid with 10×10×12.5 mm3 nominal voxel
size, 100×100×100 mm3 FOV, and 50×60×35 mm3 VOI.

In vivo 1H MR
spectra obtained using 3D MRSI sequence with 10×10×12.5 mm3
voxel size in left (red rectangle) and right (green rectangle) hypothalamus
of patients with early MS stage (MS) and healthy controls (CON). On T1-weighted
MP-RAGEs is displayed positioning of required voxels in
jSIPRO with appropriate spectra LCModel-fits.
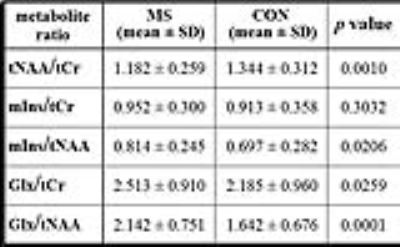
Relative concentrations (mean ± SD) of metabolite
ratios evaluated in left and right hypothalamus of patients with early MS stage
(MS, n = 31) and healthy controls (CON, n = 31). P-values expressing statistical differences in metabolite ratios between MS
and CON group performed using paired-samples
two-sided t-test in SPSS.