5269
Diffusion Tensor Imaging of Excised Rat Nerve Following Transection and Surgical Repair1Vanderbilt University Institute of Imaging Science, Vanderbilt University Medical Center, Nashville, TN, United States, 2Plastic Surgery, Vanderbilt University Medical Center, Nashville, TN, United States, 3Biomedical Engineering - School of Engineering, Vanderbilt University Medical Center, Nashville, TN, United States
Synopsis
Traumatic peripheral nerve injury (TPNI) from crushing and/or transection can lead to nerve degeneration distal to the site of injury and a temporary loss in sensorimotor function. In this study, we present our findings showing how high-resolution DTI and Tractography measurements of traumatic nerve injury in the sciatic nerve region are capable to identify and characterize nerve injury degeneration/regeneration and injury type in rats. Our findings suggest that DTI and Tractography are viable biomarkers of nerve regeneration and can provide with valuable information in the evaluation of therapeutic interventions
Introduction
Traumatic peripheral nerve injury (TPNI) from crush and/or transection can lead to nerve degeneration distal to the site of injury and a temporary loss in sensorimotor function. For function to be restored, nerve regeneration must occur in a timely fashion. Previous work has shown that diffusion tensor imaging (DTI), which provides a quantitative assay of tissue microstructure by monitoring the effect of tissue barriers on the random movements of water molecules, may be a viable biomarker of nerve regeneration interventions1-5. Here, we i) develop a DTI protocol for excised rat nerve following crush or transection/repair that will be used to evaluate nerve degeneration and/or regeneration and ii) validate these measures against behavioral measures.Methods
This study was performed using a rat sciatic nerve injury model (Sprague-Dawley) with sham surgeries (n = 21, nerve freed proximally and distally without damaging), crush injuries (n = 23), and complete transections with surgical repair (cut/repair, n = 19). Crush nerves exhibit distal Wallerian degeneration and fully recover, while only 40% of cut/repair nerves recover6. Behavioral tests with foot fault (FF) asymmetry and sciatic function index (SFI) were performed before surgical intervention, three days after surgery, and weekly thereafter for comparison to MRI. MRI studies were performed on nerves at 1, 2, 4, or 12 weeks after surgery/injury. Following euthanasia, ≈1-cm segments of the sciatic nerve was excised from the hindlimb, fixed, washed in PBS to remove excess fixative, and doped with 1mM of Gd-DTPA to reduce scan times. MRI data were then acquired in a 7-T, 16-cm bore Varian Direct Drive scanner and 25-mm quadrature Doty Scientific. DTI was performed using a 3D pulsed-gradient spin-echo (PGSE) sequence with 20 diffusion directions and b=2000 s/mm2 (δ/Δ=4/12 ms). Additional parameters included: TE/TR=22/425 ms, FOV=6x6x16 mm3, resolution=125x125x372 mm3, NEX=2, and a scan time=7 h, 40 min. Voxel-wise diffusion tensor and metrics (FA, AD/RD/MD) were estimated from these data using in-house written code (MATLAB). Region-of-interest (ROI) of the nerve were then drawn manually to estimate slice-wise FA values. Finally, DTI fiber tracts were reconstructed using ExploreDTI.Results
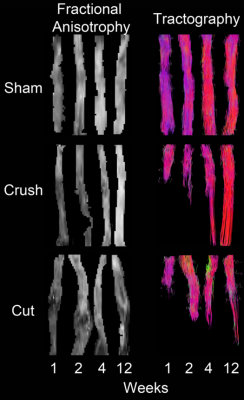
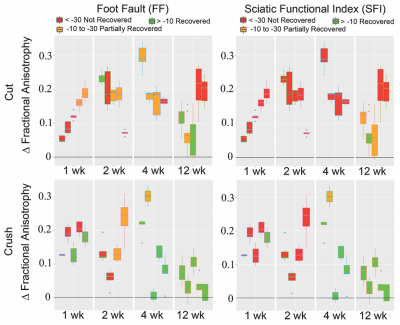
Maps of reconstructed FA and fiber tracts for nerves 1, 2, 4, and 12 weeks after TPNI are shown in Fig. 1. Diffusion Tensor Tractography (DTT) in sham nerves indicate that all fibers remain intact, while FA values indicate a homogenous reduction in FA at early time points after the sham surgery that may be indicative of inflammation/edema. For crush nerves, note the larger decrease in FA values distal to the injury site, which gradually recovers over 12 weeks. FA values in the proximal region of crush nerves were similar to FA values in sham nerves; therefore, these larger FA changes in the distal region are likely indicative of distal nerve degeneration. DTT of crush nerves were in agreement with these findings, showing continuous recovery over time. For cut/repair nerves, note the larger reductions in distal FA values and slower recovery relative to crushed nerves. DTT shows that recovery of the fibers starts at week four and even though we can see substantial recovery in week twelve, full recovery is never achieved. Figure 2 quantifies the recovery/non-recovery of each nerve in the distal region by normalizing distal FA values relative to the proximal region of the same nerve, which partially removes the confounding influence of edema at the early time points. The result, ΔFA, for cut and crush nerves are shown and color-coded using the behavioral data Foot Fault (FF) and Sciatic Function Index (SFI). At weeks 4 and 12, a clear correlation between ΔFA and the behavioral assessments can be seen for cut and crush nerves.Discussion and Conclusions
These results indicate that high-resolution DTI and fiber tracking of rat sciatic nerve ex vivo are predicative of functions (FF and SFI) and, therefore, be viable biomarkers of nerve degeneration/regeneration following TPNI/surgical repair. Furthermore, these findings suggest that we can differentiate crush (fully recover) from cut/repair (partially recover) injuries via DTI once the edema has subsided, but before getting any insight from the behavioral data. Future work includes i) relating these findings to histological measures of myelin and axon density and ii) investigating advanced diffusion models that account for edema and/or restricted diffusion in axons (e.g., diffusion kurtosis).Acknowledgements
R01 NS097821 and MR 150075 funding.References
1. Lehmann, H. C., et al. Diffusion tensor imaging to assess axonal regeneration in peripheral nerves. Exp Neurol. 2010; 223: 238–244.
2. Morisaki, S. et al. In vivo assessment of peripheral nerve regeneration by diffusion tensor imaging. J. Magn Reson Imaging. 2011; 33: 535–542.
3. Stanisz, G. J., et al. MR properties of rat sciatic nerve following trauma. Magn Reson Med. 2001; 45: 415–20.
4. Boyer, R. B. et al. 4.7-T diffusion tensor imaging of acute traumatic peripheral nerve injury. Neurosurg Focus. 2015; 39: 1–9.
5. Takagi, T. et al. Visualization of peripheral nerve degeneration and regeneration: monitoring with diffusion tensor tractography. Neuroimage. 2009; 44: 884–892.
6. R.M.R McAllister et. al. The epidemiology and management of upper limb peripheral nerve injuries in modern practice. J Hand Surg Br. 1996; 21: 4-13.
Figures