5266
Diffusion Changes in Normal-Appearing White Matter in Tracts Affected by White Matter Hyperintensities1Centre for Clinical Brain Sciences, University of Edinburgh, Edinburgh, United Kingdom, 2UK Dementia Research Institute, University of Edinburgh, Edinburgh, United Kingdom, 3Alzheimer Scotland Dementia Research Centre, University of Edinburgh, Edinburgh, United Kingdom, 4Department of Psychology, University of Edinburgh, Edinburgh, United Kingdom
Synopsis
White matter hyperintensities (WMH) are common in older brains. We analyzed how WMH affect white matter (WM) tracts and particularly their normal-appearing WM (NAWM). We used MRI of 52 participants (72.2±0.7y) to quantify diffusion parameters of WMH-affected tracts. The intersections of tracts with WMH were identified and volumes quantified. Diffusion parameters were measured for tract-WMH, tract-NAWM, and for tract-NAWM at different distances from the tract-WMH edge, and from the edge of nearby—non-intersecting—WMH. Tract-NAWM showed a gradient of diffusion abnormalities away from tract-WMH, and nearby-WMH. Tract-WMH diffusion, and either tract-WMH volume or whole-brain WMH load, predicted tract-NAWM diffusion.
Introduction
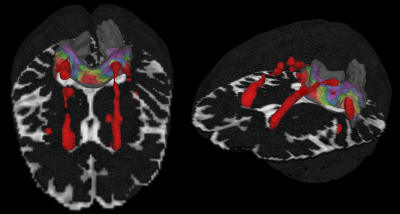
White matter hyperintensities (WMH) are common in older brains and may contribute to age-related cognitive decline. The presence of WMH within white matter (WM) tracts indicate underlying microstructural changes that could ultimately lead to cortical disconnection. Whole-brain studies of WMH also observed tissue damage in nearby normal-appearing-white-matter (NAWM)1,2, but no study has looked at the spread of damage specifically within tracts. Also, it is not established whether NAWM is affected only by the WMH intersecting the tracts (tract-WMH), or also by nearby WMH (nearby-WMH) that do not intersect. Here, we use a subsample of the Lothian Birth Cohort 19363 (LBC1936) to investigate microstructural changes of WMH-affected tracts by measuring diffusion parameters in tract-WMH, tract-NAWM, and tract-NAWM at several distances from the WMH (Fig. 1).Methods
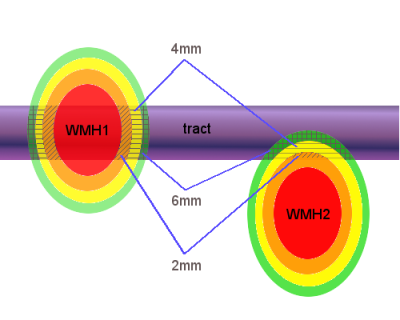
Fifty-two participants (27 male; age 72.2±0.7 years) were selected from the LBC1936 study3 to represent all levels of WMH burden (Fazekas). All patients received T1, T2, T2*-weighted imaging, FLAIR and diffusion MRI (64 directions; b= 1000 s/mm2) scans at 1.5T (GE). WMH and NAWM were segmented4 and registered to diffusion space. Fractional anisotropy (FA) and mean diffusivity (MD) maps were calculated from diffusion data (FSL v4.1). Eighteen WM tracts were reconstructed using automated tractography (Tracula5, Freesurfer v5.3) and intersected with WMH and NAWM to establish tract-WMH and tract-NAWM. We calculated volumes and weighted-mean FA and MD for each. Additionally, FA and MD were measured for tract-NAWM spatial contours at 2mm, 4mm, 6mm, 8mm and 10mm around the tract-WMH and nearby-WMH (Fig.2). For each tract and for all tracts combined (averaged for each participant), FA and MD were compared between tract-WMH and tract-NAWM; between tract-WMH and tract-NAWM at 2mm, between 2mm and 4mm, etc (SPSS21.0). We also compared the parameters from tract-WMH spatial contours with the contours established for nearby-WMH. Additionally, we investigated predictive effects of tract-WMH % volume, Fazekas and tract-WMH diffusion on tract-NAWM diffusion.Results
The tracts affected by WMH to a larger extent were the
forceps major, superior longitudinal fasciculi and the anterior thalamic
radiations, while the lowest % overlap with WMH were in the dorsal cingula and
the forceps minor. The expected patterns of decreased FA and increased MD were
generally observed in tract-WMH in comparison with tract-NAWM (in 89% of
individual tracts for FA and 94% for MD). Additionally, combining all tracts,
tract-NAWM FA was predicted by tract-WMH FA (Bstd= 0.337, t(49)=3.396, p<0.002)
and tract-WMH % volume (Bstd=-0.580, t(49)=-5.842, p<0.001) with no
significant effect of Fazekas, age or gender. Tract-NAMW MD was predicted by
tract-WMH MD (Bstd=.472, t(49)= 4.148, p<0.006) and Fazekas (Bstd=0.327,
t(49)=2.875, p<0.001), with no significant effect of tract-WMH % volume, age
or gender. We found
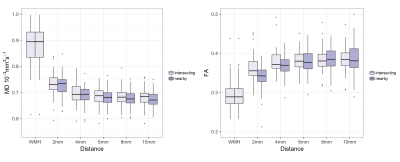
that diffusion abnormalities decreased with distance from the tract-WMH, with
lower FA and higher MD in tract-WMH compared with tract-NAWM at 2mm, in 2mm
tract-NAWM compared with 4mm, and for MD only in 4mm tract-NAWM compared with
6mm (Fig.3). This was similarly observed in 61% of individual tracts for FA and
83% for MD. A similar distance pattern was observed for nearby-WMH contours,
with generally slightly more abnormal diffusion values than in tract-WMH
contours.
Discussion
Tract-WMH showed microstructural changes suggestive of tissue damage. These were, to a lesser extent, also observed in tract-NAWM which became less pronounced along the tract and further away from the WMH. FA was predicted by %WMH overlap with the tract, while MD was predicted by whole-brain WMH burden. This suggests that tract-NAWM changes in water content and mobility, reflected by MD, are related to diffuse WM damage typically seen in the ageing brain, whereas tract-NAWM changes in myelin and axonal packing, reflected by FA, are more related to tract-specific WM damage. The changes in tract-NAWM diffusion with distance displayed similar patterns for both tract-WMH and nearby-WMH, with a comparable generally gradual decrease of abnormalities as distance increased. This may suggest that age-related WM damage does not propagate predominantly along tract axons, as expected from ischemic damage, but also propagates via different mechanisms.Conclusion
Overall, these results suggest that tract-NAWM is also affected by the pathological process underlying WMH. The changes in tract-NAWM may predate lesion progression, and may play an important role in cognitive ageing. Our future efforts are aimed at elucidating lesion progression, and the relationship between microstructural changes of tract-WMH and tract-NAWM, and cognitive functioning, at older ages.Acknowledgements
No acknowledgement found.References
1Maillard
P, Fletcher E, Harvey D, et al. White matter hyperintensity penumbra, Stroke,
2011, 42:1917–1922. 2Muñoz Maniega S, Valdés Hernández, MC, Clayden,
JD et al. White matter hyperintensities and normal-appearing white matter
integrity in the aging brain, Neurobiol. Aging, 2015, 36:909-918. 3Deary IJ, Gow AJ, Taylor
MD, et al. The Lothian Birth Cohort 1936: a study to examine influences on
cognitive ageing from age 11 to age 70 and beyond, BMC Geriatrics, 2007, 7:28. 4Valdés
Hernández MC, Ferguson KJ, Chappell FM and Wardlaw, JM. New multispectral MRI
data fusion technique for white matter lesion segmentation: method and
comparison with thresholding in FLAIR images, Eur. Radiol 2010, 20:1684-91.
5Yendiki, A, Panneck, P, Srinivasan, P, et al. Automated
probabilistic reconstruction of white-matter pathways in health and disease
using an atlas of the underlying anatomy. Front. Neuroinform, 2011, 5:23.
Figures