5261
The contribution of astrocytic aquaporin-4 to gray and white matter diffusion ansiotropy1Pediatrics, University of California Irvine, Irvine, CA, United States, 2Basic Sciences, Loma Linda University, Loma Linda, CA, United States, 3UMR 5287-Institut de Neurosciences Cognitives et Intégratives d'Aquitaine, Université de Bordeaux, Bordeaux, France
Synopsis
We investigated the impact of water channels (aquaporin 4; AQP4) on DTI metrics after silencing expression of AQP4 in the juvenile brain. We observed a significant reduction in AQP4 expression after RNA silencing of AQP4 (siAQP4) along with significantly altered DTI metrics in the cortex but not the corpus callosum (CC). No changes in cellular constituents were found. Histological studies have reported decreased AQP4 expression in acquired brain injuries. Thus, our novel findings suggest that reductions in AQP4 expression may underlie the changes in DTI metrics that are often reported in DTI studies of stroke, traumatic brain injury and others.
Introduction
Diffusion tensor imaging (DTI) has revolutionized the ability to examine the structural integrity of the brain. DTI has been predominately used to examine white matter (WM), in part, due to cohesive axonal/myelin pathways. In contrast, the diffuse gray matter (GM) is composes of neuronal cell bodies, dendrites and axons as well as attendant astrocytes and other supporting cells. The complex interface between neurons and astrocytes in GM and how diffusing water molecules report their structure from DTI is currently unknown. Aquaporin-4 (AQP4) is a water channel that is strategically located on astrocytes and is known to facilitate water movement in the brain parenchyma. We have previously demonstrated that inhibiting AQP4 expression reduces bulk water interchange in the brain assessed with diffusion weighted imaging (DWI) 1. Here we hypothesized that astrocytic AQP4 expression actively contributes to the DTI signal in GM/WM the juvenile rat brain.Methods
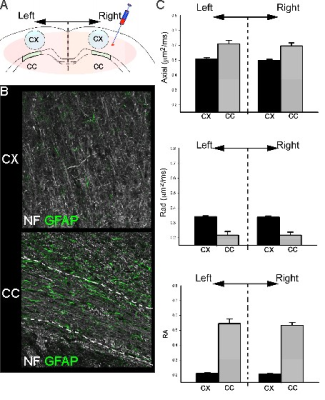
All animal experiments and care were in compliance with federal regulations and approved by the LLU IACUC. Juvenile male Sprague- Dawley rats (PND17/18; Harlan) were assigned to two groups: 1) non-targeted silencing RNA (siRNA) (Control, siGlo, n=6) or 2) siAQP4 for knockdown of AQP4 expression (n=6). Animals underwent two stereotaxic surgeries 48 hrs apart during which 4µl of siGlo or siAQP4 was injected into the right cortex 1mm lateral to bregma and 1mm into the cortex 1. 3 days after injection of siGlo/siAQP4 (PND 20/21) rat pups underwent transcardial perfusion with 4% PFA to fix tissue for ex vivo MRI and immunohistochemistry. Ex vivo DTI data were acquired on an 11.7T Bruker Advance MRI with a spin echo diffusion sequence (TR/TE = 2000/30.1 ms; one b-of 43.3 and b of 2013.2 s/mm2 in 6 equally spaced encoding directions). Parametric DTI maps were generated using in house MatLab software. Regions of interest using Cheshire software were manually drawn bilaterally in the cortex (Ctx, 5mm2) and corpus callosum (CC, 1.5mm2) on two MRI slices adjacent to the injection site. Parametric maps for Axial, radial (Rad), and Trace diffusivity (10×-5 μm2/ms) and relative anisotropy (RA) data were extracted and summarized in Excel. Standard immunohistochemistry protocols for glial fibrillary astrocyte protein (GFAP), neurofilament 200 (NF200), microglia (IBA1), vessels (tomato lectin) and AQP4 were performed.Results
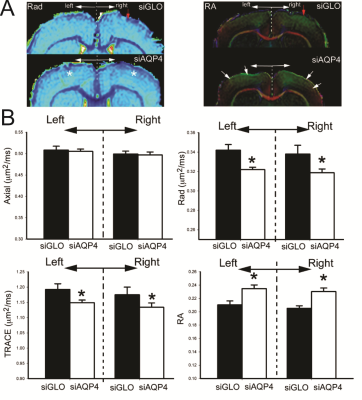
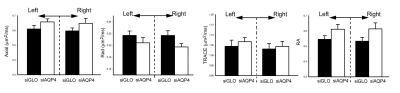
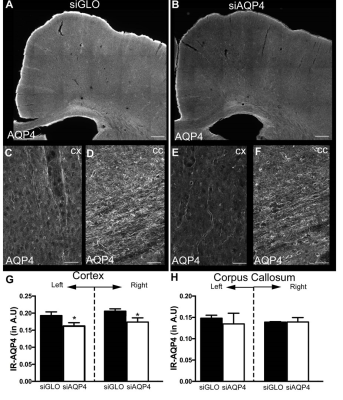
Injection of non-targeted siRNA (siGlo) into the cortex resulted in no significant changes in DTI parameters (Axial, Rad, RA; Fig 1). In contrast, siAQP4 injections resulted in significant decreases in cortical Rad and Trace with concomitant increases in RA (Fig 2). This contrasts with no significant changes in DTI measures within the CC although there were trends (Fig 3). AQP4 staining and quantification confirmed significant decrements in AQP4 expression in the cortex but not in the CC (Fig 4). In addition, we confirmed that there were no changes in astrocyte staining (GFAP), no changes in microglial density (IBA1), no changes in axonal density (NF200) and no changes in tomato lectin. The immunohistochemical data support a targeted decrease in AQP4 expression but without altering astrocytes, microglial, axonal or vessel constituents.Conclusion
The current work is demonstrates that decreasing AQP4 expression is associated with an increase in RA but without major changes in cell structures in the GM. Our findings would suggest that AQP4, and its modulation, may underlie the molecular mechanisms of DTI anisotropy transformations. It is well established that acquired brain injuries resulted in altered AQP4 expression (most notably increases) 2,3,4. Thus, our results likely impact the clinical interpretation of DTI findings in pathological conditions as water channels (AQP4) can significantly alter water diffusion in the brain parenchyma.Acknowledgements
National Institute of Child Disorders (NICHD) R01HD061946 and Swiss Science Foundation FN 310030_135617 provided support.References
1. Badaut J, Ashwal S, Adami A, et al. Brain water mobility decreases after astrocytic aquaporin-4 inhibition using RNA interference. J Cereb Blood Flow Metab 2011;31(3):819-831.
2. Badaut J, Ashwal S, Obenaus A. Aquaporins in cerebrovascular disease: a target for treatment of brain edema? Cerebrovasc Dis 2011;31(6):521-531.
3. Hubbard JA, Szu JI, Binder DK. The role of aquaporin-4 in synaptic plasticity, memory and disease. Brain Res Bull 2017.
4. Stokum JA, Mehta RI, Ivanova S, Yu E, Gerzanich V, Simard JM. Heterogeneity of aquaporin-4 localization and expression after focal cerebral ischemia underlies differences in white versus grey matter swelling. Acta Neuropathol Commun 2015;3:61.
Figures