5258
ASSOCIATION OF MID-LIFE VASCULAR RISK FACTORS AND LATE-LIFE WHITE MATTER MICROSTRUCTURE IN COGNITIVELY NORMAL OLDER WOMEN1Department of Medicine and Radiology, University of Melbourne, Royal Melbourne Hospital, Parkville, Australia
Synopsis
In this study, we explored the use of diffusion imaging measures, as possible biomarkers in clinical trials. We examined the association between vascular risk factors and white matter microstructure in normal aging. Consequently, we studied the relationship between composite and individual mid-life vascular risk factors with late-life white matter microstructure in a cohort of cognitively normal women. The results showed no association between composite score and microstructure. However, there was a significant association between systolic blood pressure and white matter microstructure such as the corpus callosum. Future work is needed to understand this relationship and its effect on cognition.
Introduction
Vascular risk factors (VRFs) have been demonstrated to be associated with subtle reductions in microstructural measures of white matter (WM) independent of WM lesions in cognitively normal older adults1. Co-occurrence of VRFs have shown to impact a change in WM microstructure over time more than individual VRFs2. A recent study showed that elevated blood pressure in mid-life and late life and elevated glucose in mid-life, but not late-life, were associated with worse late-life WM microstructure3. Another study showed that mid-life composite cardiovascular risk score (Framingham cardiovascular risk profile - FCRP) is the most important predictor of low FA and high MD in a predominantly male cohort (349 participants, 305 males)4. Our earlier study found no association of mid-life FCRP and late-life amyloid burden5. In this study, we have explored the association of mid-life vascular risk measures (FCRP and individuals VRFs) and late-life white matter microstructure in cognitively normal older women.Methods
The Women’s Healthy Ageing Project (WHAP) is a longitudinal study of the menopausal transition in cognitively-normal Australian women. Enrolment commenced in 1991. Clinical and cognition data was acquired over 20 years. 96 participants had MRI scans in 2012 (69.59 ± 2.39 years, MMSE = 28.39 ± 1.68) and mid-life FCRP (5.7 ± 5.5% measured at 49.44 ± 2.32 years). The mid-life individual VRFs included systolic blood pressure (SBP), smoking status, treatment to hypertension, diabetes status, total cholesterol and high-density lipoprotein (HDL). The MR data was acquired on a Siemens 3.0T Tim Trio, at the Radiology Department, Royal Melbourne Hospital. Diffusion MRI images were acquired using the protocol: TR/TE = 8700/92 ms, FOV 240 x 240 mm, acquisition matrix 96 x 96, b = 1000 s/mm2, voxel size 2.5 x 2.5 x 2.5 mm, 30 directions. 3D FLAIR images were collected with isotropic 1mm voxel, (TR=5000ms, TE=355ms, flip angle=120 deg, TI=1800ms). Diffusion MRI data was pre-processed using FSL DTIFIT6. The 4D diffusion tensor was converted to DTI-TK format, and a study-specific, unbiased, longitudinal tensor template constructed. This template was integrated with FSL TBSS6 to generate 4D DTI images for all patients. WMH were manually segmented from the flair images and checked by a qualified neuroradiologist. A Lesion probability map (LPM) was generated from all 96 participants and ³5% voxels were excluded from the analysis to restrict the diffusion measures to within “normal appearing” WM7. FSL Randomise was used to examine association in fractional anisotropy (FA), radial diffusivity (RD), mean diffusivity (MD), and axial diffusivity (AD) with FCRP adjusted for age. The results were corrected for Family-Wise Error (FWE) at p < 0.02, and obtained by performing voxel-wise statistics using Threshold-Free Cluster Enhancement (TFCE). To identify the area of significant correlation, the study-specific template was registered to the FSL FMRIB58-FA_1mm image using non-linear registration via ANTS8, and the ICBM DTI-81 white matter atlas9 used to identify the significant regions. Further, to examine the contribution of individual VRFs, the analysis was repeated using age, systolic blood pressure (SBP), smoking status, treatment to hypertension, diabetes status, total cholesterol and high-density lipoprotein (HDL) as separate covariates in the model.Results
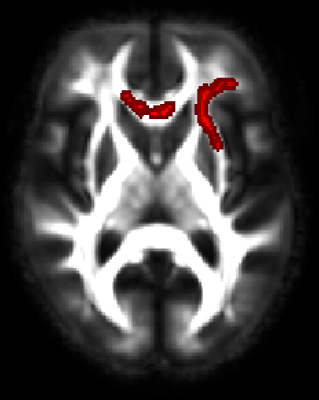
No significant association of diffusion measures was found between mid-life composite cardiovascular risk score (FCRP) with “normal appearing” white matter diffusion metrics. Mid-life age was a significant predictor of late-life WM microstructure (for all the DTI measures). No significant association between diffusion measures (FA, MD and AD) and FCRP measures was found. Mid-life systolic blood pressure was positively associated (Figure.1) with RD in the genu/body of the corpus callosum, superior longitudinal fasciculus, bilateral anterior and superior corona radiata, bilateral anterior thalamic radiation and external capsule.Conclusion
Epidemiological studies10 have shown that midlife VRFs can increase the susceptibility to Alzheimer’s disease in cognitively normal subjects. Diffusion tensor imaging–based measures may be appropriate risk-stratification tools or surrogate outcomes in clinical trials to lower vascular risk factors. Our results show that a composite vascular risk score was not associated with late-life WM microstructure in this female cohort. It is therefore important to understand the association of individual midlife VRFs to late-life WM microstructure as measured by DTI. Elevated midlife SBP was found to be strongly associated with increases in the radial diffusivity of the genu/body of the corpus callosum late-life which may reflect extended demyelination in this area. Further work is needed to understand the contribution of specific WM tracts3,11 and VRFs to age-related cognitive changes.Acknowledgements
This project was funded by Victorian Health Promotion Foundation (VicHealth) Collaborative Research Program Grant and National Health and Medical Research Council (Grants 547600, 1032350 & 1062133).References
1. O’Sullivan M, Summers PE, Jones DK, Jarosz JM, Williams SC, Markus HS. Normal-appearing white matter in ischemic leukoaraiosis: a diffusion tensor MRI study. Neurology. 2001;57:2307-2310. doi:10.1212/WNL.57.12.2307.
2. Maillard P, Carmichael OT, Reed B, Mungas D, DeCarli C. Cooccurrence of vascular risk factors and late-life white-matter integrity changes. Neurobiol Aging. 2015;36(4):1670-1677. doi:10.1016/j.neurobiolaging.2015.01.007.
3. Power MC, Tingle J V., Reid RI, et al. Midlife and late-life vascular risk factors and white matter microstructural integrity: The atherosclerosis risk in communities neurocognitive study. J Am Heart Assoc. 2017;6(5). doi:10.1161/JAHA.117.005608.
4. Zsoldos E, Jenkinson M, Filippini N, et al. Mid-life composite markers of biological ageing predict structural integrity measures in later life . In: OHBM. ; 2017.
5. Yates P, Rowe C, Villemagne V, et al. Midlife vascular risk and late-life amyloid burden: Data from the women’s healthy ageing project (WHAP). Alzheimer’s Dement. 2013;9(4):P544-P545. doi:10.1016/j.jalz.2013.04.300.
6. Smith SM, Jenkinson M, Johansen-Berg H, et al. Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31(4):1487-1505. doi:10.1016/j.neuroimage.2006.02.024.
7. Bisecco A, Caiazzo G, d’Ambrosio A, et al. Fatigue in multiple sclerosis: The contribution of occult white matter damage. Mult Scler J. 2016;22(13):1676-1684. doi:10.1177/1352458516628331.
8. Avants B, Epstein C, Grossman M, Gee J. Symmetric diffeomorphic image registration with cross-correlation: Evaluating automated labeling of elderly and neurodegenerative brain. Med Image Anal. 2008;12(1):26-41. doi:10.1016/j.media.2007.06.004.
9. Mori S, Oishi K, Jiang H, et al. Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. Neuroimage. 2008;40(2):570-582. doi:10.1016/j.neuroimage.2007.12.035.
10. Tolppanen A-M, Solomon A, Soininen H, Kivipelto M. Midlife Vascular Risk Factors and Alzheimer’s Disease: Evidence from Epidemiological Studies. J Alzheimer’s Dis. 2012;32:531-540. doi:10.3233/JAD-2012-120802.
11. Gons RAR, Van Oudheusden LJB, De Laat KF, et al. Hypertension is related to the microstructure of the corpus callosum: The RUN DMC study. J Alzheimer’s Dis. 2012;32(3):623-631. doi:10.3233/JAD-2012-121006.