5254
Characterization of white matter structures growth in common marmosets1Department of Physiology, Keio University, Tokyo, Japan, 2Central Institute for Experimental Animals, Kawasaki, Japan, 3Laboratory for Marmoset Neural Architecture, Brain Science Institute RIKEN, Saitama, Japan, 4Okinawa Institute of Science and Technology Graduate University, Okinawa, Japan, 5Department of Radiological Sciences, Tokyo Metropolitan University, Tokyo, Japan
Synopsis
This study investigated developmental patterns of white matter structures in common marmosets using DTI and MTR. Longitudinal MRI was performed to 23 marmosets at the age of 1-34 months. Tract-based ROIs were created for assessment of major fiber bundles. Population growth trajectories of association fibers estimated using Gompertz function showed different developmental patterns. As previously reported, inferior longitudinal fasciculus (ILF) showed earlier maturation with slow speed, whereas inferior fronto-occipital fasciculus (IFOF) showed slower maturation with fast speed observed in MTR, RD and FA. It indicates ILF might mature compared with IFOF at birth, which was consistent with human studies.
INTRODUCTION
Although the number of studies focusing on brain development in common marmosets (marmosets) are increasing, white matter development including myelination process in marmosets has not been well studied. For further understanding of its maturation process, MRI studies can provide macroscopic information such as mapping of the developmental changes of major white tracts, which would help efficient micro- mesoscopic investigation for the white matter development.The purpose of this study is to characterize the growth patterns of white matter tracts in common marmoset brain using multiple parameters (MTR; magnetic transfer ratio, RD, radial diffusivity, FA, fractional anisotropy) which indirectly measure the maturation of tissue microstructure.
METHODS
Twenty-three healthy common marmosets (male 11, female 12) between the age of 1 month to 34 months were utilized for this study. Longitudinal MRI data (DWI and MTR image) were obtained from these animals ranged from 3 to 8 times on 7.0 T Biospec 70/16 scanner (Bruker BioSpin:Ettlingen, Germany).
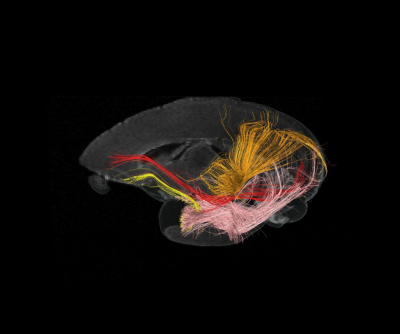
Since there were few WM (white matter) regions were available in marmosets, tract-based ROI was created. Several major white matter tracts were delineated using tractography (e.g., inferior longitudinal fasciculus, inferior fronto-occipital fasciculus) (Fig. 1) at the standard space. After transforming the ROI from standard to the individual native space, the value of MTR, FA and RD were measured.
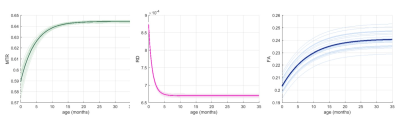
The population growth trajectories for respective regions were estimated using nonlinear mixed model, given the previous study found that the developmental pattern of global white matter development was rapid increase and stabilized thereafter1. The growth patterns of individual tract-based ROI were compared based on the parameters of the Gompertz function which represent asymptote, delay, and speed2.
RESULTS
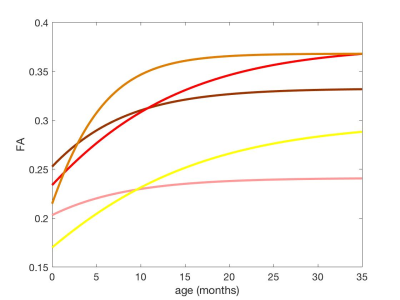
All structures showed the similar changing process; reaching the plateau after the increase (MTR and FA) or decline (RD), thus the Gompertz curve was well-fitted to MTR, RD and FA data. The differences in parameters could describe regional characteristics of their growth patterns.
In the inferior-occipital-frontal fasciculus, the delay parameter was high compared
with other regions, indicating this region might mature slowly. On the other
hand, the parameter related to speed was low, indicating the growth speed was
relatively fast. These features were consistent among MTR, RD and FA.
The feature of the inferior longitudinal fasciculus was earlier maturation, as
the delay parameter was low among
MTR, RD and FA. The maturation speed was slower especially RD and FA.
The fornix also showed the earlier maturation among MTR, RD and FA. However,
the growth speed was different between MTR and FA. Slight change of the MTR
value was observed throughout 30 months, whereas the change of RD was observed
until 7 months.
DISCUSSION
We could characterize the growth patterns of different white matter structures ranged from 1 month to 34 months, which covered the whole developmental process. The timing of maturation and accelerated speed were varied among regions. The maturation patterns of investigated regions were consistent with human studies previously reported.The inferior longitudinal fasciculus, which connects temporal and occipital area and is associated with visual processing, is known to be matured earlier3. The speed was relatively slow and it matured earlier in marmosets as well, suggesting it may be matured at birth. On the other hand, FA continued to change until adolescence at inferior fronto-occipital fasciculus in human studies3, which was consistent in marmosets, as FA increased until the age of 20 months.
The trajectories of three parameters, MTR, RD, and FA were similar but the opposite pattern also existed as observed in the fornix. Although it is not surprising as each parameter evaluate different properties of the white matter (i.e., measure of macromolecular protons or of diffusion patterns, which reflect interactions with obstacles), it needs to be elaborated to assess which aspect of white matter development these parameters evaluate.
CONCLUSION
This study revealed that marmosets also showed normal growth patterns of major white matter structures were region-specific. By detailed comparison of growth trajectories across the species including human, it is possible to characterize what may be marmoset-specific features. Such evaluation is crucial to study brain disease models and apply findings to human studies.Acknowledgements
No acknowledgement found.References
- Seki F, Hikishima K, Komaki Y, et al. Developmental trajectories of macroanatomical structures in common marmoset brain. Neuroscience. 2017; 364: 143-156.
- Sadeghi N, Prastawa M, Fletcher, et al. Regional characterization of longitudinal DT-MRI to study white matter maturation of the early developing brain. Neuroimage. 2013; 68: 236-247.
- Label C, Walker L, Leemans A, et al. Microstructural maturation of the human brain from childhood to adulthood. Neuroimage 2008; 40: 1044-1055.
Figures