5252
Ultra-High Gradient Diffusion MRI Reveals Distinct Microstructural Changes in Diffuse Gliomas Before and After Radiation Therapy1Massachusetts General Hospital, Boston, MA, United States, 2Athinoula A. Martinos Center for Biomedical Imaging, Charlestown, MA, United States, 3Computer Assisted Clinical Medicine, Medical Faculty Mannheim, Heidelberg University, Mannheim, Germany
Synopsis
The lack of a sensitive imaging method capable of capturing the full extent of glioma cell infiltration represents a significant challenge to accurate treatment planning and monitoring of therapeutic response. Here, using a recently developed diffusion MRI method (Linear Multi-Scale Model; LMM), we estimated the changes in restricted, hindered, and free water in six glioma patients pre- and post-treatment. We found scan-to-scan reproducibility of diffusion profiles in normal brain and identified distinct diffusion profiles in the tumor and peritumoral regions at different time points, thus highlighting the robustness of the LMM and its feasibility in the clinical setting.
Introduction
The
infiltrating nature of gliomas, particularly into the peritumoral area, is a
major barrier to improving clinical outcome as microscopic disease remains even
after apparent gross total resection1. T1/post-contrast and T2/FLAIR sequences are used for
surgical and radiation (RT) planning but do not capture full tumor extent2. Diffusion MRI is a more sensitive technique for in vivo characterization of tissue
microstructure. The Linear Multi-Scale Model (LMM)3 provides estimates of volume fractions of restricted,
hindered, and free water over a spectrum of length scales and orientation
distributions, thereby affording insights on water distribution in the
intracellular, extracellular, and CSF compartments. We previously showed that
the LMM framework is able to distinguish between normal brain and pathologic
tissue3,4. In this prospective study, we
apply the LMM to a cohort of glioma patients and study the longitudinal changes
in diffusion profiles in the tumor, peritumoral region, and contralateral white
matter (WM) and cortex as patients undergo treatment with RT and chemotherapy (temozolomide
(TMZ) or procarbazine, lomustine, and vincristine (PCV)).
Methods
Study design: Following IRB approval, patients with non-enhancing FLAIR-hyperintense lesions suspicious for diffuse gliomas were scanned at four time points: pre-surgery, at least 4 weeks post-surgery, at least 4 weeks post-RT, and after 3 cycles of chemotherapy.
Data acquisition: Patients were scanned on a dedicated high-gradient 3T MRI scanner (MAGNETOM CONNECTOM, Siemens Healthcare) with maximum gradient strength of 300 mT/m and maximum slew rate of 200 T/m/s5. Sagittal 2-mm isotropic resolution diffusion-weighted spin echo EPI images were acquired using simultaneous multislice (SMS) imaging5 and zoomed/parallel imaging6 for high-resolution whole-brain coverage. The following parameters were used: δ/∆=8/19, 8/49ms, 4-5 diffusion gradient increments linearly spaced from 55-293 mT/m per ∆, TE/TR: 77/3600ms, GRAPPA acceleration factor R=2, and SMS MB factor=2. Diffusion gradients were applied in 32-64 non-collinear direction with interspersed b=0 images every 16 directions. The maximum b-value at the longest diffusion time was 17,800 s/mm2. Additionally, T1-MPRAGE and T2-SPACE-FLAIR sequences were obtained. Total acquisition time was 56 minutes.
Data analysis: Following pre-processing to correct for gradient non-linearity, motion artefacts, and eddy currents7, we used spherical harmonics expansion of order 6/8 with Laplace-Beltrami regularization (λ=0.006)8 to interpolate the diffusion signal on each q-shell. The LMM of restricted, hindered, and free diffusion compartments of different sizes was obtained by concatenating a non-Gaussian diffusion response function for restricted water within cylindrical structures and a Gaussian diffusion response function for hindered and free water3. To obtain the orientation distribution functions and corresponding volume fractions, the multi-scale deconvolution inverse problem was solved by standard least-squares estimation with Tikhonov regularization. Using manual segmentation and excluding necrotic, hemorrhagic, and resected areas, the FLAIR-hyperintense region was defined as the tumor region of interest (ROI). Radiographically normal-appearing brain up to 1 cm around the FLAIR-hyperintense region was defined as the peritumoral ROI. Volume fractions of water (VFW) in the restricted, hindered, and free compartment within the tumor, peritumoral ROI, and contralateral WM and cortex were obtained by averaging volume fraction estimates over all voxels.
Results
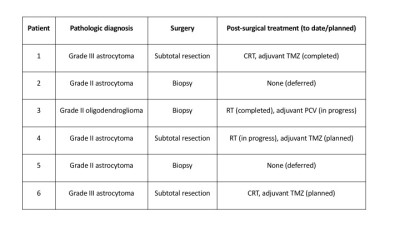
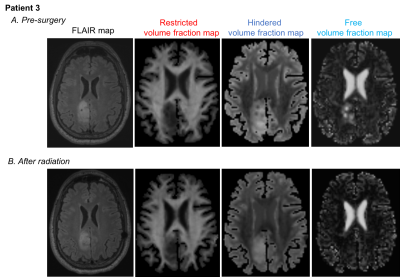
Six patients with histologically confirmed isocitrate
dehydrogenase (IDH)-mutant gliomas (Figure
1) underwent MRI scanning pre-surgery. Four patients had post-surgical and two
subjects had post-surgical and post-treatment scans. In all patients, the pre-surgical
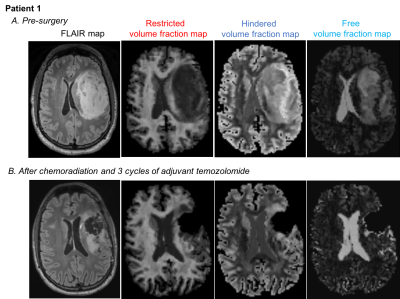
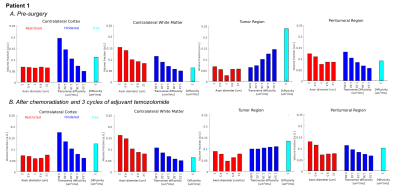
tumor ROI demonstrated increased hindered and decreased restricted water
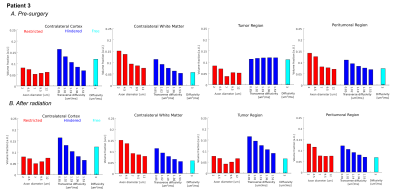
compared to normal brain (Figure 2A/3A/4A/5A). The peritumoral ROI showed a
hindered water distribution similar to normal WM and a restricted water
distribution similar to normal WM and tumor (Figure 3A/5A). Post-treatment,
the hindered VFW within the tumor was decreased in Patient 1 (Figure 2B/3B) and
similar to normal WM in Patient 3 (Figure 4B/5B). The restricted VFW was
slightly increased in Patient 1 (Figure 2B/3B) but unchanged in Patient 3
(Figure 4B/5B). When comparing normal brain within patients pre- and post-treatment,
the diffusion profiles were almost identical between scans.
Discussion
Using ultra-high gradient diffusion MRI and the
LMM, we detect distinct diffusion patterns in the tumor and peritumoral ROI not
seen on anatomic MRI. The scan-to-scan reproducibility of diffusion profiles in
normal brain within patients underscores the robustness of the LMM framework. Pre-treatment,
less restricted diffusion and higher hindered diffusion within the tumor may
reflect increased tissue permeability, permitting intracellular water to
diffuse into the extracellular space4. Higher hindered
diffusion may also be explained by increased edema. Post-treatment, less
hindered water within the tumor may indicate tissue normalization and treatment
response. Histologic validation studies are underway.
Conclusion
Our study demonstrates that the LMM framework
is a robust method, as supported by scan-to-scan reproducibility within
patients, and enables detection of distinct microstructural diffusion changes
in IDH-mutant gliomas pre- and
post-treatment.
Acknowledgements
No acknowledgement found.References
1. Giese A, Bjerkvig R, Berens ME, Westphal M. Cost of migration: invasion of malignant gliomas and implications for treatment. J Clin Oncol. 2003;21(8):1624-1636.
2. Brandsma D, van den Bent MJ. Pseudoprogression and pseudoresponse in the treatment of gliomas. Curr Opin Neurol. 2009;22(6):633-638.
3. Wichtmann B, Fan Q, Witzel T, Gerstner ER, Golby AJ, Rosen BR, Schad L, Wald LL, Nummenmaa A. Linear Multi-Scale Modeling of diffusion MRI data: A framework for characterization of orientational structures across length scales. ISMRM 24th Annual Meeting and Exhibition; 2016; Singapore.
4. Wichtmann B, Fan Q, Witzel T, Gerstner ER, Golby AJ, Santagata S, Rosen BR, Schad L, Wald LL, Huang SY. Investigating micostructural signatures for low-grade gliomas using Linear Multi-scale Modeling of diffusion MRI data. ISMRM 25th Annual Meeting and Exhibition; 2017; Honolulu, HI.
5. Setsompop K, Kimmlingen R, Eberlein E, et al. Pushing the limits of in vivo diffusion MRI for the Human Connectome Project. Neuroimage. 2013;80:220-233.
6. Eichner C, Jafari-Khouzani K, Cauley S, et al. Slice accelerated gradient-echo spin-echo dynamic susceptibility contrast imaging with blipped CAIPI for increased slice coverage. Magn Reson Med. 2014;72(3):770-778.
7. Fan Q, Nummenmaa A, Witzel T, et al. Investigating the capability to resolve complex white matter structures with high b-value diffusion magnetic resonance imaging on the MGH-USC Connectom scanner. Brain Connect. 2014;4(9):718-726.
8. Descoteaux M, Angelino E, Fitzgibbons S, Deriche R. Regularized, fast, and robust analytical Q-ball imaging. Magn Reson Med. 2007;58(3):497-510.
Figures