5249
Effects of early alcohol exposure on functional organization and microstructure of a visual-tactile integrative circuit1Department of Diagnostic Radiology and Nuclear Medicine, University of Maryland School of Medicine, Baltimore, MD, United States, 2Department of Pediatrics, University of Maryland School of Medicine, Baltimore, MD, United States
Synopsis
Children with fetal alcohol spectrum disorders (FASD) often have deficits associated with multisensory processing. Because ethanol disrupts activity-dependent neuronal plasticity, a process that is essential for refining connections during cortical development, we hypothesize that early alcohol exposure results in alterations in multisensory cortical networks, which could explain the multisensory processing deficits seen in FASD. Here, we use a gyrencephalic animal model to test the prediction that early alcohol exposure alters the functional connectivity and microstructural features of a visual-tactile integrative area with resting-state functional magnetic resonance imaging and diffusion kurtosis imaging.
Introduction
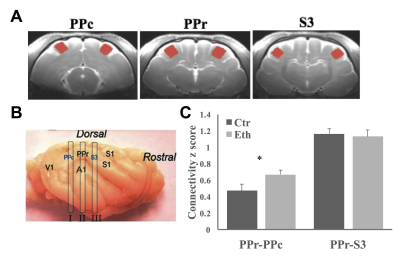
Maternal alcohol consumption during pregnancy leads to variable effects in exposed children, such as learning and memory deficits, hyperactivity and attention problems, and social and emotional problems. There is growing evidence indicating that alterations in multisensory integration (MSI) largely contribute to those problems and deficits. Ferrets are good models for developmental studies since their first postnatal month is equivalent to part of the second and the entire third trimester of human gestation. The ferret rostral posterior parietal cortex (PPr) receives convergent inputs from different visual and somatosensory sources and displays robust MSI1. In this study, we applied resting-state functional magnetic resonance imaging (rsfMRI) and diffusion kurtosis imaging (DKI) to investigate the effects of developmental alcohol exposure on the functional connectivity and microstructural features among PPr and its major input regions (i.e., caudal posterior parietal cortex (PPc) and tertiary somatosensory cortex (S3)) in ferrets. Ethanol is known to severely affect activity-dependent neuronal plasticity in the cortex, a process that is related to the refinement of connections that occurs during the third trimester of human gestation2,3. Because multisensory integration relies on the precise wiring of individual sensory systems and the accurate convergence of these systems on multisensory processing areas, we hypothesize that the ferret PPr will exhibit aberrant functional connectivity and microstructural features after early ethanol exposure.Materials and Methods
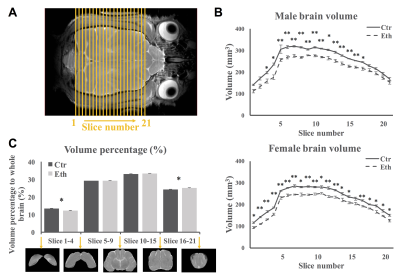
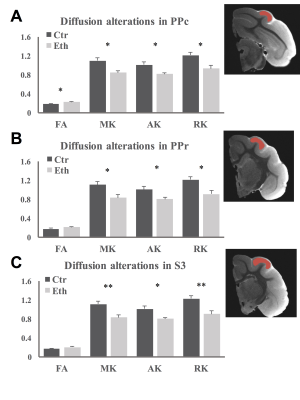
Ferret pups were given 3.5g/Kg i.p. ethanol or saline every other day between postnatal day (P) 10 to P30, which is similar to the third trimester of human gestation regarding cortical development. In vivo anatomical MRI and rsfMRI involved 8 control and 8 ethanol exposed animals. Ex vivo DKI involved 6 control and 6 ethanol animals. All animal procedures conformed to NIH guidelines and were approved by the University of Maryland, Baltimore, Institutional Animal Care and Use Committee. All in vivo MR experiments were performed between P40-50 on a Bruker Biospec 7T scanner (Bruker Biospin MRI GmbH, Germany). Light anesthesia was achieved with intraperitoneal injection of dexmedetomidine (0.03mg/Kg) before and 0.3% isoflurane during the imaging session. A MR compatible small-animal monitoring and gating system (SA Instruments, Inc., New York, USA) was used to monitor animal respiration rate and body temperature which was maintained at 37-38.5°C using a warm water bath circulation. Anatomical images (TR/TEeff = 4500/23.22ms, FOV = 45 × 45mm2, slice thickness = 1mm, in-plane resolution = 0.15 × 0.15mm2, slice number = 26) were obtained using a two-dimensional rapid acquisition with relaxation enhancement (RARE) sequence in coronal view. rsfMRI was acquired matching the anatomic images using a single shot, GE-EPI sequence (TR/TE = 1140/21.46ms) with an in-plane resolution of 0.55 × 0.55mm2. For the DKI experiments, adult animals were transcardially perfused with saline followed by 4% paraformaldehyde. Ex vivo DKI data were acquired on half of the brain using a single shot, SE-EPI sequence (TR/TE = 500/31ms, isotropic resolution = 0.25mm). Two b-values (2000 s/mm2 and 4000 s/mm2) and 64 gradient directions were used following the acquisition of five images acquired at b = 0 s/mm2. Brain volume was measured by slices (Fig 1A) and by sections (occipital, posterior parieto-temporal, anterior parieto-temporal and frontal areas). Functional connectivity between PPr and PPc, PPr and S3 were estimated. Diffusion parameters were extracted from PPc, PPr, and S3. Between groups comparisons were made using a two-sample t-test.Results and Discussion
Our study revealed that overall brain volume decreased in ethanol exposed animals (Fig 1B). However, the relative volume of occipital area decreased and that of the frontal areas increased (Fig 1C). Furthermore, we observed increased functional connectivity between PPr and PPc (Fig 2). This hyper-connectivity can be a result of deficits in neocortical plasticity4,5, specifically, reduced pruning as observed in autism6,7. The functional connectivity between PPr and S3 was not altered in our study which may be due to the fact that the somatosensory cortex develops before the visual cortex and the timing of ethanol exposure used here might affect more the latter then the former. DKI results revealed increased FA in PPc while a widespread decrease in diffusion kurtosis in all regions (Fig 3), indicating less tissue complexity which occurs in conditions such as less cell packing and less maturation8.Conclusion
In this study, we observed alterations in functional connectivity and microstructure integrity of cortical areas involved in multisensory process. The findings indicate altered neuronal plasticity and neural maturation following early ethanol exposure that may lead to disruptions in multisensory regions.Acknowledgements
This study was supported by NIH/NIAAA grant AA13023.References
1 Foxworthy, W. A., Allman, B. L., Keniston, L. P. & Meredith, M. A. Multisensory and unisensory neurons in ferret parietal cortex exhibit distinct functional properties. Eur J Neurosci 37, 910-923, doi:10.1111/ejn.12085 (2013).
2 Rema, V. & Ebner, F. F. Effect of enriched environment rearing on impairments in cortical excitability and plasticity after prenatal alcohol exposure. J Neurosci 19, 10993-11006 (1999).
3 Medina, A. E., Krahe, T. E., Coppola, D. M. & Ramoa, A. S. Neonatal alcohol exposure induces long-lasting impairment of visual cortical plasticity in ferrets. J Neurosci 23, 10002-10012 (2003).
4 Medina, A. E. & Krahe, T. E. Neocortical plasticity deficits in fetal alcohol spectrum disorders: lessons from barrel and visual cortex. J Neurosci Res 86, 256-263, doi:10.1002/jnr.21447 (2008).
5 McCool, B. A. Ethanol modulation of synaptic plasticity. Neuropharmacology 61, 1097-1108, doi:10.1016/j.neuropharm.2010.12.028 (2011).
6 Tang, G. et al. Loss of mTOR-dependent macroautophagy causes autistic-like synaptic pruning deficits. Neuron 83, 1131-1143, doi:10.1016/j.neuron.2014.07.040 (2014).
7 Rinaldi, T., Perrodin, C. & Markram, H. Hyper-connectivity and hyper-plasticity in the medial prefrontal cortex in the valproic Acid animal model of autism. Front Neural Circuits 2, 4, doi:10.3389/neuro.04.004.2008 (2008).
8 Cheung, M. M. et al. Does diffusion kurtosis imaging lead to better neural tissue characterization? A rodent brain maturation study. Neuroimage 45, 386-392, doi:10.1016/j.neuroimage.2008.12.018 (2009).
Figures