5238
Deep learning based segmentation of cardiomyocytes to aid numerical simulations of diffusion cardiovascular magnetic resonance1Aeronautics, Imperial College London, London, United Kingdom, 2National Institute of Health, Bethesda, MD, United States, 3CMR Unit, Royal Brompton Hospital, London, United Kingdom
Synopsis
To better understand how the underlying microstructure and pathology affect the DT-CMR signal in vivo, more realistic numerical models that account for irregular myocyte configurations such as sheetlets are necessary. We manually segmented cardiomyocytes from pig histology and confirmed that the resulting substrate is representative of the local microstructure through automatic segmentation of the surrounding tissue with a convolutional neural network. Monte Carlo random walk simulations, covering short and long mixing times and varying compartment diffusivities, show a mismatch between the results for the histology-based substrate and a simple cuboid model with comparable ECV and mean cell size.
Introduction
Diffusion tensor cardiovascular magnetic resonance (DT-CMR) is unique in that it enables non-invasive inference of the underlying cardiac microstructure. Recent work1,2,3 has demonstrated abnormalities in DT-CMR parameters related to sheetlet orientation for hypertrophic and dilated cardiomyopathy patients. However, the exact relationship between measured DT-CMR signal and underlying pathology is not yet fully understood.
Numerical simulations are a promising approach to synthesise the DT-CMR data of a known microstructure, thus allowing for the in silico investigation of physiological changes such as myocyte hypertrophy or disarray. Current methods4,5 model cardiomyocytes as well-defined shapes in regular arrangements, which lack the sheetlet structure found in the myocardium. We have developed simulations using a histology-based virtual microstructure and show here that this simulation substrate is representative of the local microstructure by using deep learning supported automatic segmentation, and contrast the simulated DT-CMR parameters to a simple cuboid model. This simulation substrate is used to investigate the effect of intra- and extra-cellular diffusivities on the DT-CMR parameters.
Methods
Pig heart histology was obtained through wide-field microscopy of 10μm thick radial—longitudinal slices from a transmural block2. The heart was arrested in a contracted (systolic-like) state and stained with Masson trichrome. A stack of 10 slices with spacing 100μm was registered rigidly and 3x3mm2 regions in the mid-myocardium were extracted for processing.
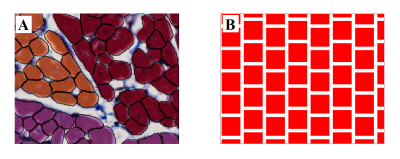
Numerical simulations were performed using an in-house Monte Carlo random walk algorithm capable of efficiently handling arbitrary cell geometries. The simulated tissue substrate is based on a subset of this histology. A 400x500μm2 region was selected and cardiomyocytes manually segmented to obtain a ground truth image (Figure 1A). Individual cells were simplified as polygons and extruded in the image-normal direction to create polyhedrons with normally distributed lengths (120μm ± 5%). This 3D tissue block was replicated to fill the imaging voxel, applying a 10deg/mm rotation along the transmural direction. A second block was created consisting of equally-sized cuboids 2μm apart, with cross-sectional area matching the mean area of the segmented cardiomyocytes (Figure 1B). Transmural rotation was applied in the same way.
We considered two values for the extra-cellular diffusivity De, 2μm2/ms and 3μm2/ms, and in each case varied the intra-cellular diffusivity Di from 0.5μm2/ms to De in steps of 0.5μm2/ms. The cell membranes were impermeable. Three pulse sequences were simulated: Stejskal–Tanner pulse gradient spin-echo (PGSE)6, second-order motion-compensated spin echo (M012-SE)7, and monopolar stimulated echo acquisition mode (STEAM)8, each with a b-value of 450s/mm2 in 6 encoding directions and a 2.8x2.8x8.0mm3 voxel.
The histology data was classified with the aid of a fully-convolutional neural network. To do so, we implemented the U-net architecture9 in the TensorFlow framework10 with 4 layers, 16 features, and 3 colour channels. The network was trained for 10 hours on the manually segmented ground truth image before evaluating the image stack. The resulting segmentation was processed to extract cells and compute their size and shape distributions.
Results
Figure 2 shows histograms comparing the cell geometry in the segmented ground truth image with the automatically-segmented surrounding tissue. The extra-cellular volume fraction was high at 34.5% and 32.0% respectively, which is attributed to fixation-induced tissue shrinkage.
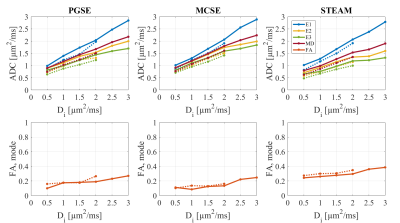
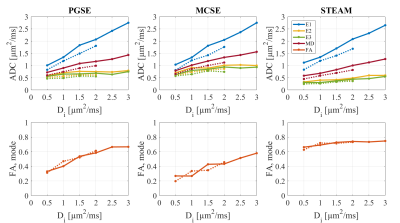
Figures 3 and 4 show the observed DT-CMR parameters as a function of Di (and De) for the two substrates. The results are separated into the three different pulse sequences.
Discussion
The size and shape distributions of the manually segmented ground truth tissue region used to create the simulation substrate show it is a good approximation to the surrounding tissue. When comparing the cuboid model to this realistic substrate, it becomes clear that the lack of sheetlets/shear layers results in a higher FA.
The automatic segmentation produces larger cells, as seen in the tail of the area distribution (Figure 2). This may be explained by the size of the segmented microscopy image (3x3mm2), which could cause a large number of cells to be cut diagonally as they rotate in the transmural direction, increasing their apparent cross-sectional area.
There is a clear dependence on the intra-cellular diffusivity but choosing a different extra-cellular diffusivity has less effect for the same Di. Both SE sequences report similar parameters, but at long mixing times (STEAM) the sequence better senses the restriction perpendicular to the myocytes’ long axis.
Conclusion
By using a realistic simulation substrate based on histology we can more reliably investigate how the DT-CMR signal is affected by changes in microstructure. A simple cuboid-based approximation of the tissue microstructure does not sufficiently represent the underlying microstructure and results in artefactual changes in the derived DT-CMR parameters. Neural-network based segmentation of histological sections allows a large number of cells to be segmented automatically and can be used to create increasingly realistic simulation substrates in the future.Acknowledgements
This work was funded (in part) by an Imperial College London British Heart Foundation Centre of Research Excellence Pilot Grant.References
- Von Deuster C, Sammut E, Asner L, et al. Studying dynamic myofiber aggregate reorientation in dilated cardiomyopathy using in vivo magnetic resonance diffusion tensor imaging. Circ Cardiovasc Imaging. 2016;9(10):e005018.
- Ferreira PF, Kilner PJ, McGill LA, et al. In vivo cardiovascular magnetic resonance diffusion tensor imaging shows evidence of abnormal myocardial laminar orientations and mobility in hypertrophic cardiomyopathy. J Cardiov Magn Reson. 2014;16:87.
- Nielles-Vallespin S, Khalique Z, Ferreira PF, et al. Assessment of Myocardial Microstructural Dynamics by In Vivo Diffusion Tensor Cardiac Magnetic Resonance. J Am Coll Cardiol. 2017;69(6):661-676.
- Bates J, Teh I, McClymont D, et al. Monte Carlo simulations of diffusion weighted MRI in myocardium: Validation and sensitivity analysis. IEEE T Med Imaging. 2017;36(6):1316-1325.
- Wang L, Zhu YM, Li H, et al. Simulation of diffusion anisotropy in DTI for virtual cardiac fiber structure. In: FIMH 2011, LNCS 6666, pp. 95-104, 2011.
- Stejskal EO, Tanner JE. Spin diffusion measurements: Spin echoes in the presence of a time-dependent field gradient. Chem Phys. 1965;42:288-292.
- Welsh CL, DiBella EVR, Hsu EW. Higher-order motion-compensation for in vivo cardiac diffusion tensor imaging in rats. IEEE T Med Imaging. 2015;34(9):1843-1853
- Reese TG, Weisskoff RM, Smith RN, et al. Imaging myocardial fiber architecture in vivo with magnetic resonance. Magn Reson Med. 1995;34:786-791.
- Ronneberger O, Fischer P, Brox T. U-net: Convolutional networks for biomedical image segmentation. In: MICCAI 2015, Part III, LNCS 9351, pp. 234–241, 2015.
- Abadi M, Agarwal A, Barham P, et al. TensorFlow: Large-scale machine learning on heterogeneous systems. 2015. Software available from tensorflow.org.
Figures
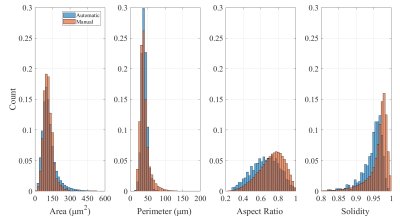
Histograms of myocyte size and shape measures. In the manually and automatically segmented images (2D pixel masks), each 4-connected region was considered a cell and processed separately. From left to right: Area - sum of pixels classifying the cell. Perimeter - number of boundary pixels, which corresponds roughly to the path length around the edge. Aspect Ratio - ratio of minor to major axis of a fitted ellipse. Solidity - ratio of area to convex area (area of the shape's convex hull).