5236
Microstructure characterisation of fixed prostate tissue using ultra high-resolution diffusion-weighted MRI1Centre for Medical Image Computing, Department of Computer Science, University College London, London, United Kingdom, 2Champalimaud Neuroscience Programme, Champalimaud Centre for the Unknown, Lisbon, Portugal, 3Center for Advanced Imaging, University of Queensland, Brisbane, Australia, 4National Imaging Facility, Queensland Node, Center for Advanced Imaging, University of Queensland, Brisbane, Australia, 5Discipline of Medical Radiation Sciences, Faculty of Health Sciences, University of Sydney, Sydney, Australia
Synopsis
This work explores ultra-high field microimaging of prostate tissue using diffusion compartment
INTRODUCTION
In clinical prostate imaging the most common model for diffusion-weighted MRI (DW-MRI) is the mono-exponential model, which gives an apparent diffusion coefficient (ADC). But ADC is limited, as it cannot discriminate the specific histological features of the tissue. More sophisticated models that account for multiple microstructure components provide better characterisation of prostate tissue. For instance, VERDICT (Vascular, Extracellular, and Restricted Diffusion for Cytometry in Tumours) MRI provides a framework combining rich DW-MRI acquisition with a multi-compartment model to give non-invasive estimates of cancer tissue microstructure. Recent work demonstrates the potential of VERDICT for prostate imaging, to differentiate cancer and benign tissue, as well as cancer grading1,2.
Several studies compare such models in terms of quality of fit and variance of the fitted parameters3,4. Some consensus exists in the models that can best describe the signal from prostate tissue, however, typical resolution of such studies is not sufficient to relate various components of these models to specific microstructure. Diffusion microimaging5 provides spatial resolution approaching the cellular scale, which can be used to investigate the diffusion properties of different tissue types as shown in6,7. In this work we use diffusion microimaging with a richer protocol, to explore various multi-compartment models and determine ones that best characterise prostate tissue.
METHODS:
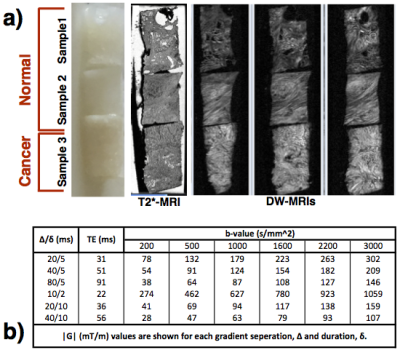
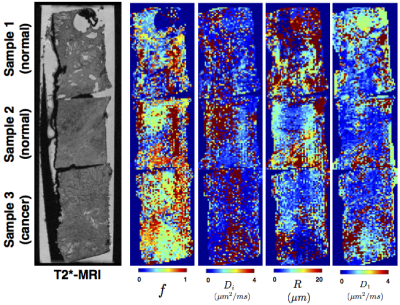
Three 3mm diameter x 4mm long samples (Figure.1a) of human prostate tissue (2 normal, 1 cancerous) were extracted from fixed radical prostatectomy specimens, with informed consent and ethics approval, as done in6.
MR microimaging
MRI data were acquired on a Bruker 16.4T AVANCE II scanner, with a rich imaging protocol (Figure 1b). The DW-MRIs were acquired with a PGSE sequence with (80μm)3 isotropic resolution, with multiple b-values and diffusion times to support estimation of various multi-compartment models. Two non diffusion-weighted images and three gradient orientations were sampled for each combination of acquisition parameters. A higher resolution (20μm)3 T1/T2*-weighted image was also acquired for anatomical reference.
Data analysis
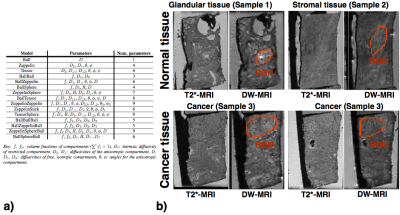
Fifteen models were fitted to the data, comprising 1, 2 or 3 compartments as listed in Figure 2a. For ex-vivo data there is no perfusion component in prostate tissue, but we tested 3-compartment models as the high-resolution data may enable separation of further non-perfusion components of the tissue. The models compared were combinations of sphere, stick, ball, and zeppelin compartments (terminology as in8), and included the conventionally used cancer models: ADC (Ball) and bi-exponential (BallBall). Model fitting was performed using the non-linear fitting procedure used in1, with data normalised using the b=0 data for each echo time, to account for T2 dependence. The models were fitted without fixing any model parameters.
Regions-of-interest (ROIs) were drawn on the DW-MRIs, by manually selecting areas composed primarily of a single tissue type, as shown in Figure.2b. We specifically included glandular and stromal tissue for normal samples and cancer tissue, by finding visual correspondence to the higher-resolution T2*-MRIs where histological features are moderately well visible (histological confirmation with stained sections is not yet available). Model fitting was done for the signal averaged over these ROIs, for all the models. The models were then ranked based on the Bayesian Information Criterion (BIC)9. Voxel-wise model fitting was also performed to extract maps of microstructure parameters.
RESULTS:
ROI analysis
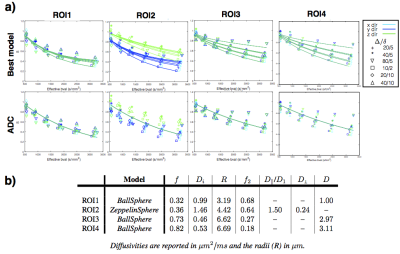
Figure 3 shows that multi-compartment models best explain the acquired data and 2-compartment models with restriction perform the best overall. The best-ranked models show that regions of cancer and glandular tissue are best described by a model with isotropic restriction, while stromal tissue requires anisotropy. The corresponding parameter estimates for the best ranking models (Figure 3b) show a higher restriction compartment in the cancer ROIs compared to normal ROIs, as expected.
Voxel-wise analysis
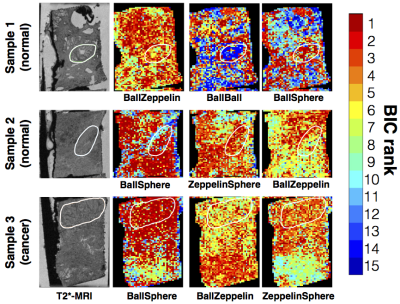
Figure 4 shows the variation of the model ranking for the top performing models in each sample. The trends are overall similar to the ROI analysis. Additionally, we find non-restriction model is sufficient in areas with lumen space. The parameter maps corresponding to BallSphere, the best performing model, is shown in Figure 5, which show plausible trends.
DISCUSSION AND CONCLUSION:
This work explores separation of the various tissue compartments in prostate samples with DW-MRIs acquired on an ultra-high field scanner. We see that with diffusion microimaging, we can explore the specific components of the microstructure using multi-compartment models. However, none of the models used here fully capture the complexity of the data (Figure 3a). Incorporating more orientations in the DW-MRI acquisitions will improve the model fitting and provide better anisotropy characterisation. Future work will also investigate more complex models, including incorporation of T2 relaxation in modelling.
A bigger set of tissue samples are required for a comprehensive analysis to determine the best model that fully characterises the microstructure of normal and cancerous prostate tissue.
Acknowledgements
This work is funded by a grant PG14-018-TR2 from Prostate Cancer UK. EPSRC grant EP/N021967/1 supports EP's work on this topic, and EP/M020533/1 and EP/N018702/1 support DCA.References
1. Panagiotaki, E. et al. Microstructural Characterization of Normal and Malignant Human Prostate Tissue With Vascular, Extracellular, and Restricted Diffusion for Cytometry in Tumours Magnetic Resonance Imaging. Invest. Radiol. 50, 218–227 (2015).
2. Johnston, E. et al. Short term repeatability of microstructural (VERDICT) MRI vs. ADC in prostate cancer. in ISMRM (2016).
3. Liang, S. et al. Information-based ranking of 10 compartment models of diffusion-weighted signal attenuation in fixed prostate tissue. NMR Biomed. 29, 660–671 (2016).
4. Bailey, C. et al. (ISMRM 2017) Validation of VERDICT MRI using fresh and fixed prostate specimens with aligned histological slices. in (2017).
5. Bourne, R., Kurniawan, N., Cowin, G., Sved, P. & Watson, G. 16 T diffusion microimaging of fixed prostate tissue: preliminary findings. Magn. Reson. Med. 66, 244–247 (2011).
6. Bourne, R. M. et al. Microscopic diffusivity compartmentation in formalin-fixed prostate tissue. Magn. Reson. Med. 68, 614–620 (2012).
7. Bourne, R. M. et al. Biexponential diffusion decay in formalin-fixed prostate tissue: Preliminary findings. Magn. Reson. Med. 68, 954–959 (2012).
8. Panagiotaki, E. et al. Compartment models of the diffusion MR signal in brain white matter: A taxonomy and comparison. NeuroImage 59, 2241–2254 (2012).
9. Schwarz, G. Estimating the Dimension of a Model. Ann. Stat. 6, 461–464 (1978).
10. Panagiotaki, E. et al. Noninvasive Quantification of Solid Tumor Microstructure Using VERDICT MRI. Cancer Res. 74, 1902–1912 (2014).
Figures