5229
Sensing Von Economo Neurons in the Insula with Multi-shell Diffusion MRI1Parietal, Inria, CEA, Université Paris-Saclay, Palaiseau, France, 2Athena, Inria, Sophia-Antipolis, France, 3Royal Institute of Technology in Stockholm, Stocholm, Sweden, 4DEFI, Inria, Palaiseau, France, 5Stanford Medical School, Palo Alto, CA, United States
Synopsis
Sensing microstructural characteristics of human brain tissue with clinical scanners has been an area of heated debate in the diffusion MRI (dMRI) community . In this work we propose that diffusion MRI on clinical scanners is sensitive to the presence of Von Economo neurons.
Von Economo neurons, located in the insular and anterior cingular cortices, are large neurons present only in mammals with high cognitive functions. Albeit these neurons' role is not yet known, evidence suggests they facilitate rapid long-range information integration.
In this work, we provide theoretical and in-silico evidence that the dMRI signal is sensitive to the presence of Von Economo neurons as well as preliminary evidence on human dMRI images.
Introduction
Sensing microstructural characteristics of human brain tissue with clinical scanners has been an area of heated debate in the diffusion MRI (dMRI) community [1,2]. We present evidence that, if we focus on the cortex, specifically in the insula and anterior cingulate cortex (ACC), the unique characteristics of the cellular population in these gyri allow using clinical-grade scanners to sense the presence of Von Economo neurons (VENs). VENs, uniquely localised in the insula and ACC, are large neurons thought to play an important role in goal-directed behaviours and emotional regulation [3]. However, there is a lack of tools enabling studies on VENs population characteristics and their link to brain function and behaviour.
We present, for the first time, evidence that VENs induce an attenuation to the dMRI signal. This opens up the possibility of testing hypotheses linking VENs to function and behaviour non-invasively in human subjects.
Experiments and Results
We first examined the feasibility of detecting diffusion in compartments of the volume of VENs, based on Jelescu et al [1, eq 37] which models diffusion transversal to the cell as within a disc. Using their parameters, the expected attenuation of the dMRI signal at 40 mT/m and a gradient pulse length (δ) of 10.6ms agreeing with the Human Connectome Project (HCP), and a very large pulse separation (Δ). We assumed VENs spherical of volume 10,000um3 [5]. The attenuation S will decay as -ln S = 2.2197. This means that we can expect a signal decay of around 89%.
Using Balinov et al [6, eq 4] long time regime for the sphere the same δ:
- -ln S = 0.4758 (37% decay, Gmax = 40 mT/m, i.e. normal clinical scanner)
- -ln S = 2.180 (88% decay, Gmax = 80 mT/m, i.e. PRISMA clinical scanner)
- -ln S = 3.674 (97% decay, Gmax = 100mT/m, i.e. HCP scanner)
Moreover, using the more appropriate Balinov model [6] for diffusion in a sphere at limited diffusion time (τ=Δ-δ/3) and the HCP acquisition parameters: Δ/δ=43.1/10.6ms, Bmax = 3000 s/mm2 (Gmax = 100 mT/m) and water diffusion constant D=2.4 um2 / ms we obtain -ln S = 1.7570 which means a signal decay of 83%. Hence, attenuation is much larger than SNR. In consequence, we can measure diffusion inside of these cells if we assume that the soma is simple enough that we can model it as an empty space.
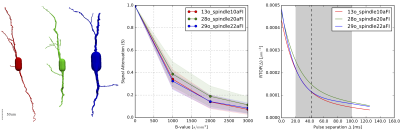
To bring our theoretical results closer to real scenarios we used 3-dimensional microscopic reconstructions of VENs (see Fig. 1, left) [7]. We simulated the dMRI signal considering the VENs as hollow, filled with water and with purely reflecting boundaries [8]. Our results back our hypothesis that the VENs, even in the case of complex geometry, induce a significant decay in the dMRI signal (Fig 1., center), specifically a 60% decay is observable at low b-values of 1000s/mm2, provided that the acquisition protocol is appropriate. Furthermore, our simulated results show that the Return-to-Origin Probability (RTOP) is appropriate for assessing cellular population characteristics in the human cortex. At limited diffusion times, RTOP is driven by the surface-volume ratio of closed compartments in the probed tissue and, as diffusion time increases, is solely driven by volume [9]. Our results show that RTOP, which we fit according to Fick et al [2], evolves over Δ with the expected convergent decay and that some VENs are distinguishable at different diffusion times (see Fig 1. right).
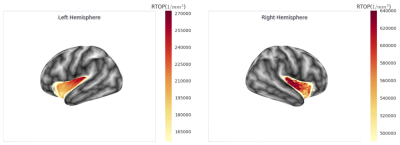
Finally, as proof of concept, we analysed the RTOP values on the human insular cortex. For this, we used a sample of the HCP dataset [10], we fit the multi-shell signal with a graphnet-regularised qτ-dMRI model [2] and then computed the RTOP at the voxel level on the insular cortex. Finally, for each subject, we projected the RTOP measures over the cortical mesh obtained through the freesurfer pipeline and averaged it across subjects (see Fig. 2). Our preliminary results show an RTOP gradient starting at the insular pole and evolving along the dorsal axis on both hemispheres. We hypothesise that this gradient is driven by the presence of the VENs and is in agreement with the granular-dysgranular characterisation of the insular cortex [3].
Discussion
We presented, evidence that VENs induce a decay on the dMRI signal. To test our hypothesis, we provided evidence from three angles: theoretical evidence based on simple signal models conceiving the VENs as spheres; simulations of the dMRI signal in microscopically-obtained 3D VEN models assuming that cell organelles don't alter the dMRI signal significantly; finally, by analysing the HCP dataset and linking RTOP, which we argue is a good subsidiary of VENs population characteristics, with current hypotheses on the neuronal layer configuration of the insula and VEN presence.
Acknowledgements
Authors would like to acknowledge support from the Inria Equipe Associée program, project Large Brain Nets, and the ERCs Advanced grant CoBCoM and Starting grant NeuroLangReferences
[1] Jelescu IO, Zurek M, Winters KV, Veraart J, Rajaratnam A, Kim NS, Babb JS, Shepherd TM, Novikov DS, Kim SG, Fieremans E (2016) In vivo quantification of demyelination and recovery using compartment-specific diffusion MRI metrics validated by electron microscopy. 132:104–114. doi: 10.1016/j.neuroimage.2016.02.004
[2] Fick RHJ, Petiet A, Santin M, Philippe A-C, Lehericy S, Deriche R, Wassermann D (2018) Non-parametric graphnet-regularized representation of dMRI in space and time. Medical Image Analysis 43:37–53. doi: 10.1016/j.media.2017.09.002
[3] Namkung H, Kim S-H, Sawa A (2017) The Insula: An Underestimated Brain Area in Clinical Neuroscience, Psychiatry, and Neurology. Trends Neurosci 40:200–207. doi: 10.1016/j.tins.2017.02.002
[5] Evrard HC, Forro T, Logothetis NK (2012) Von Economo Neurons in the Anterior Insula of the Macaque Monkey. Neuron 74:482–489.
[6] Balinov B, Jonsson B, Linse P, Soderman O (1993) The NMR Self-Diffusion Method Applied to Restricted Diffusion. Simulation of Echo Attenuation from Molecules in Spheres and between Planes. 104:17–25. doi: 10.1006/jmra.1993.1184
[7] Allman JM, Tetreault NA, Hakeem AY, Manaye KF, Semendeferi K, Erwin JM, Park S, Goubert V, Hof PR (2010) The von Economo neurons in frontoinsular and anterior cingulate cortex in great apes and humans. Brain Struct Funct 214:495–517. doi: 10.1007/s00429-010-0254-0
[8] Nguyen DV, Li J-R, Grebenkov D, Le Bihan D (2014) A finite elements method to solve the Bloch–Torrey equation applied to diffusion magnetic resonance imaging. Journal of Computational Physics 263:283–302. doi: 10.1016/j.jcp.2014.01.009
[9] Schwartz LM, Hürlimann MD, Dunn K-J, Mitra PP, Bergman DJ (1997) Restricted diffusion and the return to the origin probability at intermediate and long times. Phys Rev E 55:4225–4234.
[10] Sotiropoulos SN, Jbabdi S, Xu J, Andersson JL, Moeller S, Auerbach EJ, Glasser MF, Hernandez M, Sapiro G, Jenkinson M, Feinberg DA, Yacoub E, Lenglet C, Van Essen DC, Ugurbil K, Behrens TEJ (2013) Advances in diffusion MRI acquisition and processing in the Human Connectome Project. 80:125–143. doi: 10.1016/j.neuroimage.2013.05.057
Figures