5224
Intra-axonal Fractional Anisotropy from Fiber Ball Imaging1Medical University of South Carolina, Charleston, SC, United States, 2Neurology, Medical University of South Carolina, Charleston, SC, United States, 3Pediatrics, Medical University of South Carolina, Charleston, SC, United States, 4Medical College, Medical University of South Carolina, Charleston, SC, United States, 5Neuroscience, Medical University of South Carolina, Charleston, SC, United States
Synopsis
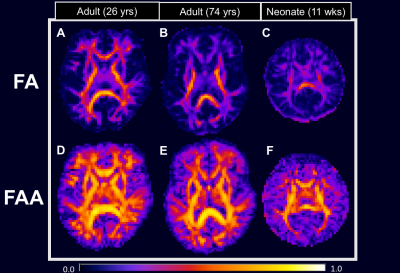
Using fiber ball imaging (FBI), we isolate the intra-axonal compartment of the white matter with strong diffusion weighting in order to suppress the extra-axonal water fraction and then calculate its associated fractional anisotropy (FA). We call this parameter the fractional anisotropy axonal (FAA) in contrast to the conventional FA, and we compare these two measures in three subjects: a healthy young adult, a cognitively intact older adult with severe white matter abnormalities, and a neonate with acute hypoxic ischemic injury. Our results indicate that FAA reveals diffusion anisotropy that is not apparent using the conventional FA.
Introduction
Fiber ball imaging (FBI),1 a diffusion MRI method, requires strong diffusion weighting in order to suppress the extra-axonal water signal thereby isolating the intra-axonal compartment. While closely related to q-ball imaging (QBI),2 FBI utilizes an inverse Funk transform to calculate the fiber orientation density function (fODF) for white matter (WM) whereas QBI uses a forward Funk transform to estimate the diffusion orientation distribution function (dODF). Typically, however, the fODF is more sensitive to resolving fiber crossings than the dODF. Additionally, FBI quantifies the microstructural parameter,$$$ \zeta = \frac{f}{\sqrt{D_a}} $$$ where $$$ f $$$ is the axonal water fraction and $$$ D_a $$$ is the intrinsic intra-axonal diffusivity.1 Here we show that FBI further provides an estimation of the intra-axonal compartment fractional anisotropy (FA), or the fractional anisotropy axonal (FAA), which is the FA if all the extra-axonal water were removed from the white matter. To illustrate the differences between the conventional FA and our novel FAA, we present preliminary results using data from a healthy young adult (26 yrs), a cognitively intact older adult (74 yrs) with severe WM abnormalities, and a neonate (11 wks) with hypoxic-ischemic-encephalopathy (HIE).Methods
FBI determines spherical harmonic expansion coefficients for the fODF from high angular resolution diffusion imaging (HARDI) data using a single b-value shell.1 The b-value should be about 4000 s/mm2 or larger in order to adequately suppress signal from extra-axonal water,3 which is neglected in the analysis. More precisely, one obtains the fODF, $$$ F( \bf n) $$$, for a direction $$$ \bf n $$$ as
$$ F({\bf n}) = \sum_{l=0}^{\infty}\sum_{m=-l}^{l} \it c_{l}^{m}\it Y_{l}^{m} \tt (\theta,\phi), $$
where $$$ \it Y_{l}^{m} $$$ are the spherical harmonics, $$$ (\theta,\phi) $$$ are the spherical angles for $$$ {\bf n} $$$, and $$$ \it c_{l}^{m} $$$ are the complex-valued expansion coefficients; because of reflection symmetry, $$$ c_{2l+1}^{m} = 0 $$$. The diffusion tensor, $$$ {\bf D}_a $$$, for the intra-axonal compartment is related to $$$ \it c_{l}^{m} $$$ by
$$ {\bf D}_a = \frac{D_a}{c_{0}^{0} \sqrt{30}} \begin{bmatrix} \frac{\sqrt{30}}{3}c_{0}^{0}-\frac{\sqrt{6}}{3}c_{2}^{0}+c_{2}^{2}+c_{2}^{-2} & ic_{2}^{2}-ic_{2}^{-2} & -c_{2}^{1}+c_{2}^{-1} \\ ic_{2}^{2}-ic_{2}^{-2} & \frac{\sqrt{30}}{3}c_{0}^{0}-\frac{\sqrt{6}}{3}c_{2}^{0}-c_{2}^{2}-c_{2}^{-2} & -ic_{2}^{1}-ic_{2}^{-1} \\ -c_{2}^{1}+c_{2}^{-1} & -ic_{2}^{1}-ic_{2}^{-1} & \frac{\sqrt{30}}{3}c_{0}^{0}+\frac{2\sqrt{6}}{3}c_{2}^{0} \end{bmatrix}. $$
Following from this, the FAA can be written as
$$ FAA = \sqrt{\frac{3\sum_{m=-2}^{2}|c_{2}^{m}|^{2}}{5|c_{0}^{0}|^{2}+2\sum_{m=-2}^{2}|c_{2}^{m}|^{2}}}. $$
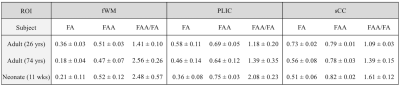
To test this expression for FAA, we acquired HARDI data at b = 6000 s/mm2 with 128 diffusion encoding directions (adults) and 64 diffusion encoding directions (neonate) using a 3T Siemens Prisma scanner. We also acquired diffusional kurtosis imaging (DKI)4 datasets with 64 directions and b = 0, 1000, and 2000 s/mm2 in order to quantify the FA. The HARDI acquisition had 3 mm isotropic voxels with TE = 110 ms (adults) and TE = 98 ms (neonate), while the DKI acquisition had 3 mm isotropic voxels with TE = 110 ms (adults) and 2.5 mm isotropic voxels with TE = 85 ms (neonate). FAA and FA maps were produced with in-house Matlab scripts and diffusional kurtosis estimator (DKE),5 respectively. Regions of interest (ROIs) sampled from the frontal WM (fWM), the posterior limb of the internal capsule (PLIC), and the splenium of the corpus callosum (sCC) were manually drawn using MRIcron6 for each map.
Results
As seen in Figure 1, the FAA (lower row) has substantially higher values in most WM regions in comparison to the FA (upper row). This suggests intra-axonal water diffusion to be more anisotropic than for the full tissue, as might be expected. Furthermore, FAA detects sizeable diffusion anisotropy in brain regions where the FA is low. This is most apparent for the older adult (Figure 1, panels B and E) and the neonate (Figure 1, panels C and F).
Table 1 shows mean ± standard deviation values for FA, FAA, and FAA/FA in three ROIs for each subject. In every case, FAA is greater than FA. The FAA/FA ratio varies across regions from 1.41 to 2.48 in the fWM, 1.18 to 2.08 in the PLIC, and 1.09 to 1.61 in the sCC.
Discussion
Expressions for $$$ {\bf D}_a $$$ and FAA were provided using FBI-derived fODF spherical harmonic coefficients. Not surprisingly, FAA was markedly higher than conventional FA for all cases, since FAA reflects purely diffusion anisotropy of water restricted to the intra-axonal compartment. However, the FAA/FA ratio displays considerable regional variability, as is most striking for the older adult with severe WM abnormalities and the neonate with HIE. This work highlights the potential of quantifying diffusion anisotropy with FAA in WM regions that normally report low FA values due to large extra-axonal water signal contributions. Furthermore, FAA may be particularly relevant to applications in demyelinating disorders or edema, where FA may be abnormally low.Acknowledgements
The Litwin Foundation (PI: Helpern)
NIH/NIA K23AG044434 (PI: Benitez)
Rare Disease Foundation (PI: Benitez)
Medical University of South Carolina Neuroscience Institute Grant (PI: Jenkins)
T32DC0014435 (Trainee: McKinnon)
T32GM008716 (Trainee: Glenn)
References
1. Jensen, J.H., G. Russell Glenn, and J.A. Helpern, Fiber ball imaging. Neuroimage, 2016. 124(Pt A): p. 824-33.
2. Tuch, D.S., Q-ball imaging. Magn Reson Med, 2004. 52(6): p. 1358-72.
3. McKinnon, E.T., et al., Dependence on b-value of the direction-averaged diffusion-weighted imaging signal in brain. Magn Reson Imaging, 2017. 36: p. 121-127.
4. Jensen, J.H., et al., Diffusional kurtosis imaging: the quantification of non-gaussian water diffusion by means of magnetic resonance imaging. Magn Reson Med, 2005. 53(6): p. 1432-40.
5. Tabesh, A., et al., Estimation of tensors and tensor-derived measures in diffusional kurtosis imaging. Magn Reson Med, 2011. 65(3): p. 823-36.
6. Rorden, C. and M. Brett, Stereotaxic display of brain lesions. Behav Neurol, 2000. 12(4): p. 191-200.
Figures