5219
The Benefits Of Cognitive Training Depend On The Wiring Of The Brain1School of Psychology, Australian Catholic University, Melbourne, Australia, 2Cardiff University, Cardiff, United Kingdom
Synopsis
There is strong evidence that task-specific training leads to changes in brain structure, as assessed using MRI-based techniques that probe microstructure or morphology. In the present study, we want to understand the specific mechanisms of action of task-specific training and identify other critical ingredients. Here, we used a well-established working memory training program and state-of-the art neuroimaging methods in 40 healthy adults. Further research on dose, timing, and duration of training is necessary to generalize the training protocols to the field of structural neuroplasticity.
Introduction.
Recent evidence has accumulated that structural plasticity occurs in response to task-specific training, both in healthy participants1,2 and patients with brain injury3. However, the exact mechanisms by which such training changes brain structure and leads to improvements in outcome is still unclear.4 Here, we explored the relationships between training-induced improvements in cognition and: (a) ‘dosage’ (training duration); and (b) the baseline topology of the brain’s wiring (using the graph-theoretical metric of global efficiency)Materials and Methods:
Participants: 40 young healthy adults (Mage=26.6 years; SD=6.46) were randomly allocated to either: (a) ‘high capacity’ working memory training (performance-related adaptive increases in task difficulty); or (b) ‘low capacity’ training (constant low level of task difficulty) for 8 weeks, using the Cogmed RM software. Cognitive performance was assessed before and after training using the Cambridge Brain Sciences (CBS) battery.5
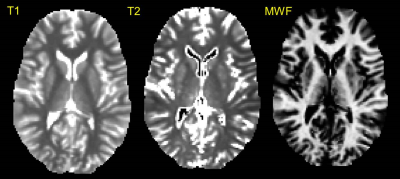
MRI: Data were acquired on a 3T GE HDx MRI system using a multi-modal microstructural imaging protocol. Whole brain 60 direction cardiac-gated HARDI data (b = 1200 s/mm2, 2.4 mm isotropic) were acquired for tractographic reconstruction, and the mcDESPOT protocol as described by Deoni et al. 6,7 was used for whole brain relaxometry (1.7mm isotropic). Finally, a whole brain 1mm isotropic FSPGR scan was used to generate an anatomical reference.
Preprocessing: The SPGR and bSSFP images for mcDESPOT were corrected for motion using FLIRT8 and the mcDESPOT model was fitted to obtain maps of myelin water fraction, T1 and T2 (subsequently used to derive R1 and R2 maps). All quantitative maps were co-registered to the anatomical reference using FNIRT. HARDI data were similarly nonlinearly registered to the anatomical reference (correcting for EPI-distortion, eddy currents and motion), and the damped Richardson-Lucy algorithm9 (dRL) used to construct whole-brain tractography.
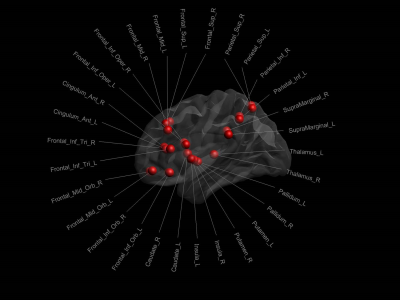
Graph theoretical analyses: Thirty regions from the automated anatomical labeling atlas10 were selected on the anatomical reference to define the nodes of the working memory network (see Figure 1) and the dRL used to identify edges of the graph. The edge-weights were then defined by the metrics derived from the mcDESPOT pipelines (Figure 2), leading to 3 different networks (each represented by a 30x30 connectivity matrix) for each participant. Finally, we quantified global efficiency as the average inverse shortest path length for each of the three weighted networks and for the two timepoints.11
Results.
Training (Cogmed) results. Participants trained extensively for eight weeks (40 sessions in total, about 45 min per session, mean total training duration=1579±39.54min, range=991-2162min). We observed significant improvements for all training tasks in the high capacity training group (all p’s<0.001).
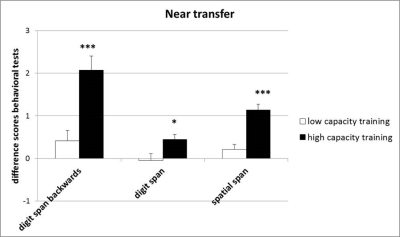
Cognitive task (CBS) results: Significant performance improvements were seen on the digit span forwards [F(1, 38)=6.04, p<0.05], digit span backwards [F(1, 38)=16.64, p<0.001], and spatial span tasks [F(1, 38)=26.89, p<0.001] in the post-training session for the high capacity training group (Figure 3).
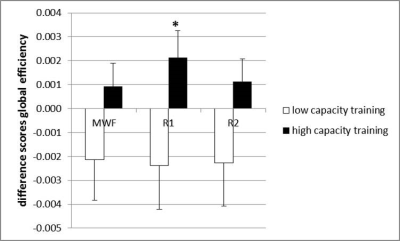
Graph theoretical results. We found a training-induced increase in global efficiency of the R1-weighted working memory network in the high capacity training group [F(1, 38)=4.50, p<0.04, Figure 4].
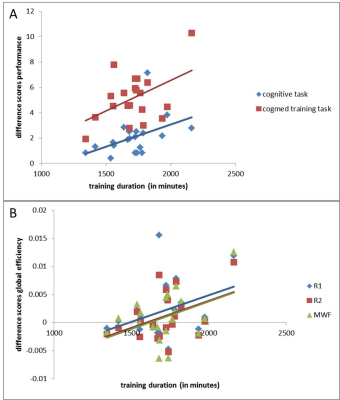
Dose-response relationships: Is more better? We observed a significant positive dose-response relationship between training-duration and improvement in cognition in the digit span backwards task (r=0.441, p<0.05), and Cogmed training tasks (e.g., inputmodule with lid, r=0.473, p<0.05). (Figure 5A). Thus, dose duration accounts for about 20% of the variance in improvement. There were only marginal positive dose-response relationships between training-duration and global efficiency (p’s<0.06-0.07, Figure 5B).
Dose-response relationships: better for whom? Controlling for training duration, significant positive correlations were found between baseline global efficiency of the R1- and R2- weighted networks (R1-WN and R2-WN) and improvements in performance on both the Cogmed training tasks (e.g., dataroom training task, r=0.616, p<0.01 for R1-WN; r=0.617, p<0.01 for R2-WN) and the CBS tests (spatial span task: r=0.536, p<0.05 for R1-WN; r=0.517, p<0.05 for R2-WN). In other words, baseline brain wiring accounts for about 30-40% of the variance in improvement.
Discussion and conclusions.
We have shown for the first time that those participants who have higher global efficiency of their working memory network at baseline, will see greater benefits of training, both in terms of improvement on the training tasks themselves but also on supplementary tests of cognition. Our data also suggest that training duration only accounts for one fifth of the variance in cognitive outcomes, and more training may be necessary to overcome working memory difficulties in clinical populations with brain injuries. Continued work in this field will hopefully see structural MRI metrics used as biomarkers to help informed stratified and personalized treatment plans at the individual patient level.Acknowledgements
This work was facilitated by an ACURF Program grant awarded to Karen Caeyenberghs by the Australian Catholic University (ACU). Moreover, this research was funded by the Wellcome Trust through a New Investigator Award (096646/Z/11/Z) and a Strategic Award (104943/Z/14/Z). We would like to thank Adam Hampshire, London, for the provision of the cognitive benchmark tests and Hadi Hosseini, Stanford University, for help with the longitudinal plugin of the GAT toolbox. The authors have no conflict of interest to declare.References
1. Thomas C, Baker CI. Teaching an adult brain new tricks: a critical review of evidence for training-dependent structural plasticity in humans. Neuroimage. 2013;73:225-236.
2. Caeyenberghs K, Metzler-Baddeley C, Foley S, et al. Dynamics of the Human Structural Connectome Underlying Working Memory Training. J Neurosci. 2016;36(14):4056-4066.
3. Caeyenberghs K, Clemente A, Imms P, et al. Evidence for training-dependent structural neuroplasticity in brain-injured patients: A critical review. Neurorehabil Neural Repair. 2017
4. Lang CE, Lohse KR, Birkenmeier RL. Dose and timing in neurorehabilitation: prescribing motor therapy after stroke. Curr Opin Neurol. 2015;28(6):549-555.
5. Owen AM, Hampshire A, Grahn JA, et al. Putting brain training to the test. Nature. 2010;465(7299):775-778.
6. Deoni SC, Rutt BK, Arun T, et al. Gleaning multicomponent T1 and T2 information from steady-state imaging data. Magn Reson Med. 2008;60(6):1372-1387.
7. Deoni SC, Mercure E, Blasi A, et al. Mapping infant brain myelination with magnetic resonance imaging. J Neurosci. 2011;31(2):784-791.
8. Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23 Suppl 1:S208-219.
9. Dell'Acqua F, Rizzo G, Scifo P, et al. A model-based deconvolution approach to solve fiber crossing in diffusion-weighted MR imaging. IEEE Trans Biomed Eng. 2007;54(3):462-472.
10. Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273-289.
11. Latora V, Marchiori M. Efficient behavior of small-world networks. Phys Rev Lett. 2001;87(19):198701.
Figures