5209
Improved fibre tracking with optimized diffusion-weighted single-refocused spin echo EPI1Brain Imaging Center (BIC), Goethe University Frankfurt, Frankfurt am Main, Germany, 2Department of Neurology, Palacky University Olomouc and University Hospital Olomouc, Olomouc, Czech Republic
Synopsis
This study investigates the suitability of data acquired with an optimized diffusion-weighted (DW) single-refocused spin-echo EPI sequence for fibre tracking. The proposed scheme uses dummy scans of 1.5s duration prior to the acquisition of each multi-slice data volume, thus driving eddy-currents into a steady-state, thus allowing to use an optimized parameter setting of the eddy-current correction tool “EDDY”. Results show that the proposed sequence yields better fibre tracking results than conventional DW sequences with a more precise estimation of the subsidiary fibre orientations, showing additional connections to the lateral frontal, parietal and temporal cortices and to the thalamus.
Introduction
Recently, an optimized diffusion-weighted (DW) single-refocused spin-echo (srSE) EPI sequence was presented, using dummy scans of 1.5s duration prior to the acquisition of each multi-slice data volume [1]. This sequence has the advantages of (1) driving eddy-currents into a steady-state, (2) avoiding slice-dependent eddy-current artifacts induced by the transition phase between different DW gradient directions, and (3) allowing to improve eddy-current compensation (ECC) via post-processing with an optimized parameter setting of the eddy-current correction tool EDDY [2]. The goal of this study was to show that the optimized DW-srSE-EPI sequence yields improved fibre tracking results, as compared to the conventional DW-srSE-EPI sequence without dummy scans [3,4] and the standard DW twice-refocused spin-echo (trSE) sequence with intrinsic ECC [5].Methods
Experiments
Five healthy volunteers were scanned on a 3T whole-body MRI-scanner, using a body TX-coil and an 8-channel phased-array head RX-coil. Parameters identical for all sequences (DW-srSE-EPI with/without dummy scans and DW-trSE-EPI) were: 60 DW gradient directions, b-value=1000s/mm2, in-plane spatial resolution=2×2mm2 (FOV=192×192mm2, matrix-size=96×96), 60 interleaved axial slices (2 mm thickness, no inter-slice gap), TR/volume=9s, TE=81/95ms (srSE/trSE), echo-spacing=0.86ms, partial-Fourier=6/8, 2-fold-acceleration (iPAT=2). For each subject, T1-weighted data with an isotropic resolution of 1mm were acquired for anatomical reference via the three-dimensional magnetization-prepared rapid gradient-echo imaging sequence [6].
Fibre tracking
Preprocessing and subsequent data analysis were performed as previously explained in detail [1]. Probability distributions of diffusion parameters and fibre orientations for probabilistic fibre tracking were estimated in each brain voxel via BEDPOSTX [7,8]. Non-brain tissue was removed from the anatomical image using BET [9]. A global affine transform between the anatomical image and the brain-extracted average undistorted reference images acquired at b=0 was calculated via FLIRT [10,11]. A cerebrospinal fluid (CSF) mask was created by segmenting the anatomical image via FAST [12] and thresholding the resulting probability map at 0.05. A fibre tracking seed mask was manually drawn inside the corpus callosum on a single sagittal slice of the anatomical image. Probabilistic tractography was performed via PROBTRACKX [7,8] on all data sets, with a total of 1000 samples (i.e., probability streamlines heading to both opposite directions) starting from each seed voxel with a step length of 0.5mm. The tracking was stopped as soon as one of the following cases occurred: (1) more than 2000 steps, (2) angle between two consecutive steps exceeding 78.5°, (3) sample leaving the brain mask, (4) sample entering the CSF mask. The sample counts per voxel were stored in the anatomical image space, yielding a connectivity distribution map per data set. The resulting maps were thresholded at 20 samples per voxel and visually compared.
Results
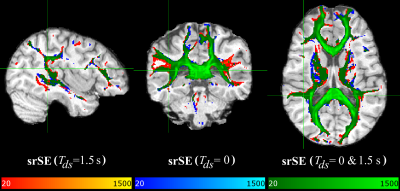
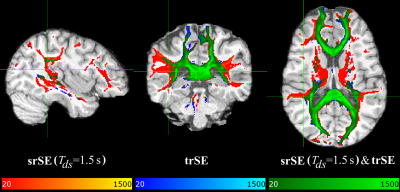
Figures 1&2 show three orthogonal planes of the resulting connectivity distribution maps, comparing the proposed sequence DW-srSE-EPI (dummy scans of 1.5s duration, optimized post-processing) with either the standard DW-srSE-EPI sequence (no dummy scans, default EDDY setting) (Fig. 1) or the standard DW-trSE-EPI sequence (Fig. 2). Colour intensities represent the number of streamlines binned in each voxel with a lower threshold of 20 fibres per voxel. The different colours denote fibre tracts that are only visible for data based on (1) the proposed sequence (red-yellow overlay), or (2) the respective standard sequence (blue overlay). The green overlay indicates voxels in which results from the proposed and the respective standard sequence overlap. All data sets yielded connectivity distribution maps of the transcallosal fibre tracts that projected to mesial parts of the cerebral cortex in both hemispheres (Figs.1&2, green). Data based on the proposed sequence showed additional connections to the lateral frontal, parietal and temporal cortices as well as to the thalamus (Figs.1&2, red), whereas the data based on the standard sequences did not show any consistent additional projections (Figs.1&2, blue).Discussion/Conclusion
The tractography results demonstrate that the reconstructed transcallosal fibre tracts extend into more lateral cortical regions for data acquired with the proposed DW-srSE-EPI sequence with dummy scans, in comparison to data based on one of the standard sequences (DW-srSE-EPI without dummy scans or DW-trSE-EPI with intrinsic eddy-current compensation). This finding agrees with results presented previously [13], demonstrating that probabilistic fibre tracking with multiple orientations outperforms the single fibre orientation approach which ignores the subsidiary fibres and detects only the prevailing fibre direction leading to the medial cortices. The higher signal-to-noise ratio provided by the proposed sequence allows for more accurate estimation of the subsidiary fibre orientations, which may considerably improve the quality and quantity of the detected fibre tracts.Acknowledgements
No acknowledgement found.References
1. Shrestha M, Hok P, Nöth U, Deichmann R (2017). Eddy current artifact reduction in diffusion-weighted single-refocused spin-echo EPI. Proc ISMRM 25: 3353.
2. Andersson JLR, Sotiropoulos SN (2016). An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. NeuroImage 125, 1063–1078.
3. Stejskal EO, Tanner JE (1965). Spin diffusion measurements: spin echoes in the presence of a time-dependent field gradient. J Chem Phys 42(1), 288-292.
4. Turner R, Le Bihan D, Maier J, Vavrek R, Hedges LK, Pekar J (1990). Echo-planar imaging of intravoxel incoherent motion. Radiology, 177(2), 407-414.
5. Reese TG, Heid O, Weisskoff RM, Wedeen VJ (2003). Reduction of eddy-current-induced distortion in diffusion MRI using a twice-refocused spin echo. Magn Reson Med 49(1), 177-182.
6. Deichmann R, Good CD, Josephs O, Ashburner J, Turner R (2000). Optimization of 3-D MP-RAGE sequences for structural brain imaging. NeuroImage 12(1), 112-127.
7. Behrens TEJ, Woolrich MW, Jenkinson M, Johansen-Berg H, Nunes RG, Clare S, Matthews PM, Brady JM, Smith SM (2003). Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn Reson Med, 50(5), 1077-1088.
8. Behrens TEJ, Berg HJ, Jbabdi S, Rushworth MFS, Woolrich MW (2007). Probabilistic diffusion tractography with multiple fibre orientations: What can we gain? NeuroImage 34, 144–155.
9. Smith SM (2002). Fast robust automated brain extraction. Hum Brain Mapp 17, 143–155.
10. Jenkinson M, Bannister P, Brady M, Smith S (2002). Improved Optimization for the Robust and Accurate Linear Registration and Motion Correction of Brain Images. NeuroImage 17, 825–841.
11. Jenkinson M, Smith S (2001). A global optimisation method for robust affine registration of brain images. Med Image Anal 5, 143–156.
12. Zhang Y, Brady M, Smith S (2001). Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging, 20(1), 45-57.
13. Jbabdi S, Sotiropoulos SN, Haber SN, Van Essen DC, Behrens TE (2015). Measuring macroscopic brain connections in vivo. Nat Neurosci, 18(11), 1546-1555.
Figures