5208
The unresolved problem of synaptic connectivity in the context of the cerebro-cerebellar loop1Department of Physics, University of Pavia, Pavia, Italy, 2Brain Connectivity Center, C. Mondino National Neurological Institute, Pavia, Italy, 3Florey Institute of Neuroscience and Mental Health, University of Melbourne, Heidelberg, Australia, 4Florey Department of Neuroscience and Mental Health, University of Melbourne, Melbourne, Australia, 5Department of Physics, University of Milan, Milan, Italy, 6Department of Brain and Behavioral Sciences, University of Pavia, Pavia, Italy, 7Queen Square MS Centre, UCL Institute of Neurology, Faculty of Brain Sciences, University College London, London, United Kingdom, 8Brain MRI 3T Research Center, C. Mondino National Neurological Institute, Pavia, Italy
Synopsis
Recently advanced tractography has been used for assessing the feasibility of characterizing cerebro-cerebellar tracts, acknowledging the issue of how tractography deals with polysynaptic connectivity in particular through the thalamus. In this work, four different synaptic connectivity configurations at thalamic level were hypothesized and each one of them was reconstructed using high-quality HCP data. Our findings revealed the key role of thalamic connectivity for characterizing the cerebro-cerebellar connections. We can hypothesize that the relation between streamlines entering/outing the thalamic synapses is multiplicative and our findings using polysynaptc thalamic tracts support the fact that cognitive/associative areas are the mainly involved in the cerebro-cerebellar connections.
Purpose
The cerebellar involvement in both cognitive and motor functions is increasingly recognized(1). Furthermore, cerebellar processing is mediated by the cerebro-cerebellar loop composed of: cerebello-thalamo-cortical (CTC) and cortico-ponto-cerebellar pathways(2). Recent investigations using advanced MRI techniques assessed the feasibility of characterizing such tracts in humans in vivo(3-5), acknowledging, but not resolving the issue of how tractography deals with polysynaptic connectivity, e.g. through the thalamus. Current tractography algorithms use deep GM masks to stop connectivity and reconstructing tracts in a mono-synaptic fashion(6). Remains unresolved how to reconstruct loops that involve several relays and polysynaptic connectivity. Here, we present possible strategies to reconstruct synaptic connectivity in the particular case of the cerebro-cerebellar loop. We investigated how to reconstruct the efferent tract, which comes from the cerebellar cortex, through the superior cerebellar peduncle(SCP) and the red nucleus(RN), reaching the thalamus and continuing towards the cerebral cortex. We hypothesized four different synaptic connectivity configurations at thalamic level, developed a pipeline to reconstruct each one of them and characterize the CTC pathways starting from the high-quality data from the Human Connectome Project(7).Methods
Subjects: 28subjects (10/18 male/female; 22-35 years) were acquired using a Siemens3T Connectome Skyra scanner. Minimal pre-processed 3DT1-weighted images and diffusion weighted data (1.25mm isotropic resolution, b=1000,2000,3000s/mm2, 90 directions) were downloaded from ConnectomeDB(http://db.humanconnectome.org).
Whole-brain tractography: Fibre-orientation distributions (FODs) were calculated using the multi-shell-multi-tissue CSD(8). Whole-brain tractogram was performed using probabilistic streamline tractography (iFOD2)(9): step=1mm, angle=45°, FOD threshold=0.1, 30 million streamlines. 5-classes segmentation was performed on 3DT1-weighted images and WM-GM interface was used for randomly seeding the streamlines within the Anatomically-Constrained Tractography framework(6).
Cerebro-cerebellar connections: An ad-hoc atlas was created in MNI152 space combining deep GM structures, AAL(10) and SUIT atlases(11). Cerebral and cerebellar cortices were parcellated according to anatomical and functional basis(12). The atlas was dilated to overlap GM-WM interface and transformed to subject-space. Afferent and efferent possible cerebro-cerebellar connections were selected from the whole-brain tractogram as those connecting cerebral cortical and subcortical structures with contralateral cerebellar cortical areas.
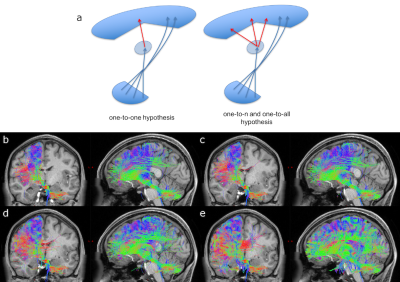
CTC identification: Two steps were implemented: 1)Cerebellar peduncles masks were created using a cerebellar WM atlas(13). RN masks were created averaging hand-drawn masks on six randomly chosen subjects. From the identified cerebro-cerebellar connections, right/left monosynaptic CTC pathways were selected by isolating streamlines passing through the SCP and contralateral RN masks and avoiding streamlines passing through the other cerebellar peduncles. 2)Some of the streamlines from step1) terminated in the thalamus. To identify the continuation of the efferent cerebellar pathway towards the cortex, the intersection between each thalamus and each CTC streamlines from step1) was used as starting point to select streamlines originating in the thalamus and reaching the ipsilateral cerebral cortex. To investigate how thalamic polysynaptic tracts may affect the CTC pathway, three different hypotheses were considered for streamlines entering/outing the thalamus: i)one-to-one relationship; ii)one-to-n relationship, n=number of ongoing streamlines greater than 1; iii)one-to-all relationship. For each overall CTC pathway, we calculated the proportion of the tract projecting to a particular cortical ROI by assessing the trGMcROI index(3). One-way ANOVA was performed among trGMcROI of all tracts.
Results
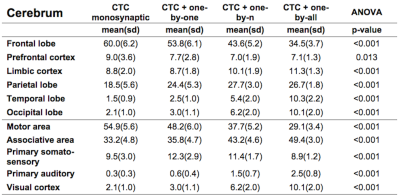
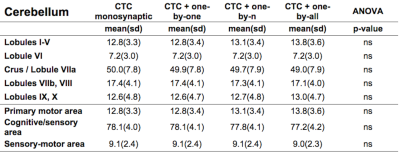
Figure1 shows both monosynaptic and polysynaptic CTC pathways reconstructed without (b) and with (c,d,e) adding thalamic streamlines, respectively. Table1 and 2 report trGMcROI for all CTC pathways with respect to cerebral and cerebellar parcellations, respectively. It's worth noting that the inclusion of connections originated in the thalamic synapses affects cerebral indices while cerebellar indices aren't affected.Discussion
Our main finding is confirming that thalamic connectivity has a fundamental influence on the characterization of the cerebro-cerebellar connections. From physiology, it is known that synapses amplify the number of entering connections, but it isn't clear which is this multiplication factor. Our results also confirm previous work(3) where the majority of the CTC streamlines projects to cerebellar cognitive/associative areas and aren't affected by thalamic synapses. If we assume that this proportion needs to be reflected also in the connectivity to the cerebral cortex, our results indicate that the most plausible option for the efferent thalamic projections are given by the one-to-n and the one-to-all strategies, which are not reconstructed by traditional tractography algorithms (Fig1c,1d and Table1). However, when considering all cortical parcellations, our work presents some differences to(3), such as in the occipital and temporal lobes. These discrepancies require further investigation as could be due to the improved image quality of the present dataset, to distortion correction and reduced partial volume effects. In conclusion, our work has highlighted possible ways to address the problem of poly-synapses in the reconstruction of cerebral loops, which produce different results depending on the assumption on thalamic synapses connectivity. Further multi-level studies are warranted to identify the actual cerebro-cerebellar connectivity and propose algorithms able to solve this major issue.Acknowledgements
Data were provided by the Human Connectome Project, WU-Minn Consortium (Principal Investigators: David Van Essen and Kamil Ugurbil; 1U54MH091657) funded by the 16 NIH Institutes and Centers that support the NIH Blueprint for Neuroscience Research; and by the McDonnell Center for Systems Neuroscience at Washington University. University of Pavia, C. Mondino National Neurological institute (Pavia, Italy), UCL-UCLH Biomedical Research Centre (London, UK), National Health and Medical Research Council of Australia, the Australian Research Council, and the Victorian Government’s Operational Infrastructure Support Grant for funding and for ongoing support.References
1. D'Angelo E, Casali S. Seeking a unified framework for cerebellar function and dysfunction: from circuit operations to cognition. Front Neural Circuits (2013) 6(116):1-23. doi: 10.3389/fncir.2012.00116.
2. Ramnani N. The primate cortico-cerebellar system: anatomy and function. Nat Rev Neurosci (2006) 7(7):511-22. doi: 10.1038/nrn1953.
3. Palesi F, Tournier J-D, Calamante F, Muhlert N, Castellazzi G, Chard D, et al. Contralateral cerebello-thalamo-cortical pathways with prominent involvement of associative areas in humans in vivo. Brain Struct Funct (2015) 220(6):3369-84. doi: 10.1007/s00429-014-0861-2.
4. Palesi F, Rinaldis AD, Castellazzi G, Calamante F, Muhlert N, Chard D, et al. Contralateral cortico-ponto-cerebellar pathways reconstruction in humans in vivo: implications for reciprocal cerebro-cerebellar structural connectivity in motor and non-motor areas. Sci Rep (2017) 7(1). doi: 10.1038/s41598-017-13079-8.
5. Kwon HG, Hong JH, Hong CP, Lee DH, Ahn SH, Jang SH. Dentatorubrothalamic tract in human brain: diffusion tensor tractography study. Neuroradiology (2011) 53(10):787-91. doi: 10.1007/s00234-011-0878-7.
6. Smith RE, Tournier J-D, Calamante F, Connelly A. Anatomically-constrained tractography: improved diffusion MRI streamlines tractography through effective use of anatomical information. NeuroImage (2012) 62(3):1924-38. doi: 10.1016/j.neuroimage.2012.06.005.
7. Van Essen DC, Smith SM, Barch DM, Behrens TEJ, Yacoub E, Ugurbil K. The WU-Minn Human Connectome Project: An overview. NeuroImage (2013) 80:62-79. doi: 10.1016/j.neuroimage.2013.05.041.
8. Jeurissen B, Tournier JD, Dhollander T, Connelly A, Sijbers J. Multi-tissue constrained spherical deconvolution for improved analysis of multi-shell diffusion MRI data. NeuroImage (2014) 103:411-26. doi: 10.1016/j.neuroimage.2014.07.061.
9. Tournier J-D, Calamante F, Connelly A, editors. Improved probabilistic streamlines tractography by 2 nd order integration over fibre orientation distributions. 2010 2010; (2010).
10. Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage (2002) 15(1):273-89. doi: 10.1006/nimg.2001.0978.
11. Diedrichsen J, Balsters JH, Flavell J, Cussans E, Ramnani N. A probabilistic MR atlas of the human cerebellum. NeuroImage (2009) 46(1):39-46. doi: 10.1016/j.neuroimage.2009.01.045.
12. Brodmann K. Brodmann’s Localisation in the Cerebral Cortex. 3rd ed: Springer Science (2006). 1-298 p.
13. Van Baarsen KM, Kleinnijenhuis M, Jbabdi S, Sotiropoulos SN, Grotenhuis JA, Walsum AMVCV. A probabilistic atlas of the cerebellar white matter. NeuroImage (2016) 124:724-32. doi: 10.1016/j.neuroimage.2015.09.014.
Figures