5207
DTI-based Network Analysis of APP/PS1 Mouse Brains with Age and Sex1Chemical & Biomedical Engineering, Florida State University, Tallahassee, FL, United States, 2Center for Interdisciplinary MR, National High Magnetic Field Laboratory, Tallahassee, FL, United States
Synopsis
This study utilizes DTI and graph theory as a novel way for identifying early pathology and connectivity changes related to Alzheimer’s Disease. As a function of phenotype, age and sex, DTI studies were performed on APP/PS1 mouse brains and age-matched wild type controls at 11.75 T. Current data shows a drop in FA and a decrease in connectivity in the temporal region of the brain. High resolution 3D images acquired at 21.1 T display the presence of amyloid plaques, which temporally correlate with the progression of structural connectivity alterations in this transgenic preclinical model.
Introduction
Alzheimer’s disease (AD) is the most common form of dementia, characterized by memory loss and changes in behavior [1]. The most prevalent preclinical model is the double transgenic mouse expressing human genes for amyloid precursor protein (APP) and presenilin-1 (PS1). Clinically, MRI is used to diagnose AD by means of volumetrics, mainly focusing on hippocampal atrophy [2]. In this study, diffusion tensor imaging (DTI) data acquired from an APP/PS1 model (5xFAD) at multiple time points is coupled with network theory to examine structural connectivity alterations as a function of age and sex compared to wild type controls. Furthermore, high field high resolution susceptibility-weighted 3D imaging was used to identify β-amyloid plaque deposition as a function of age and sex at early points in pathological progression.Materials and Methods
Image datasets were acquired using preserved mouse brains (4% paraformaldehyde) from male and female specimen (5xFAD) either expressing the APP/PS1 phenotype or an age-matched wild type. Brains were harvested at 1, 2, 4 and 6 months (N=5 per age, sex and phenotype). Using an 11.75-T magnet, DTI data was acquired with a multi-slice 2D spin echo using 18 diffusion encoding directions (nominal b = 400 s/mm2, Δ=21ms and δ=3ms) and four unweighted acquisitions. Two to three brains were imaged simultaneously with an in-plane resolution of 100x100 μm, matrix size of 256x256, slice thickness of 500μm, repetition time of 2 s and echo time of 30 ms. With 15 averages and a 47-h total acquisition time, high signal-to-noise ratios of 60:1 were obtained. To detect β-amyloid plaques, 3D images were acquired at 11.75 and 21.1 T using a true 3D gradient-recalled echo sequence having an echo time of 15 ms and repetition time of 100 ms to achieve an isotropic 25-μm resolution in approximately 14.5 h. DTI data were analyzed using DSI Studio [3] to generate tracts using the following parameters: Threshold=0.10, Angular Threshold=60, Step Size=0.05mm, Minimum Length=1mm, Maximum Length=25mm, and termination after 106 seeds. Network analysis was performed in a binary fashion on these tracts by imputing determined adjacency matrix into MATLAB and extracting graph properties including: local and global efficiency, clustering coefficients, and characteristic path lengths. Using network matrices, the software package Gephi yielded graph properties and provided a visual representation of networks. Graph properties were statistically compared to each other within age and transgenic groups and against wild-type controls.Results and Discussion
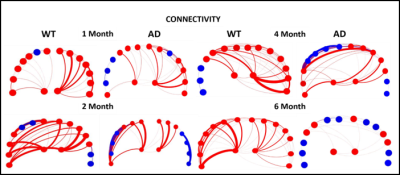
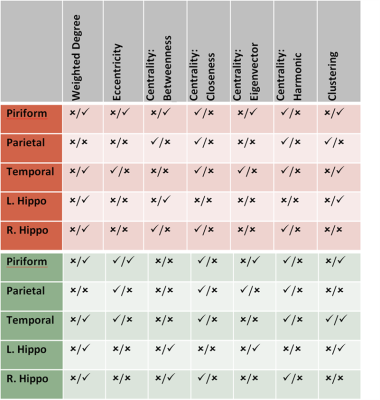
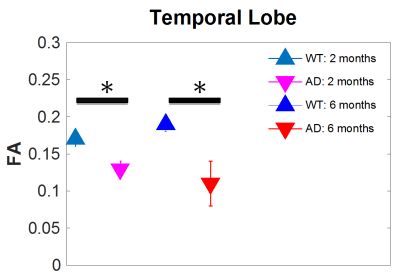
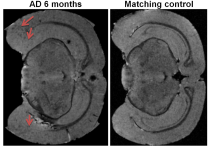
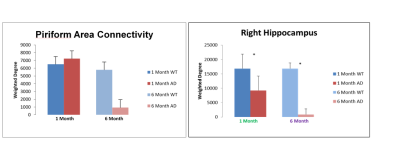
Five main areas of focus were the Piriform Area, Temporal Lobe, Parietal Lobe, and Left and Right Hippocampus. Current data from preserved 5xFAD mice indicate a significant decrease in FA in the temporal cortex. Structural connectivity work was performed in DSI Studio utilizing 14 equally spaced prolate spheroidal regions of interest (ROIs) that were positioned in cortical regions and manually segmented nodes representative of the left and right hippocampus to quantify connectivity both locally and globally [Figure 1]. Tracts were run to each individual node in order to derive quantifiable adjacency matrices that correspond to neural graphs. Graph theoretical analysis used a binary approach to study the structural connectivity in the models. Impacts of aging can be seen in closeness and harmonic centrality. Weighted degree and clustering in older samples show impacts of phenotype. In younger samples, impacts of phenotype can be seen in Eccentricity as well as closeness and harmonic centrality [Figure 2]. Additionally, we detected significant increase in the characteristic path length between the young controls and young Alzheimer’s. Current findings show a significant decrease in the FA in the temporal regions of the cortex in transgenic models [Figure 3]. Extracellular plaques detected using the 21.1-T scanner without contrast agents are localized in regions with FA decreases [Figure 4]. Least significant difference and one way ANOVA were used to determine statistical significance among samples.Conclusion
This research could be utilized as a method of identifying Alzheimer’s pathology and other neurodegenerative diseases, or classifying progression, earlier than is currently possible in patients using network theory and MRI. This will allow patients to receive treatment earlier, possibly before symptoms present and provide caretakers with time to prepare. This will reduce patient and family costs and emotional distress associated with care of AD and other neurodegenerative diseases. Additionally this work will help to expand the application of DTI and network theory to identification and progression of other neurodegenerative diseases.Acknowledgements
NSF (DMR-1157490)
State of Florida
National High Magnetic Field Laboratory User Collaborations Grant Program
References
1. U.S Department of Health & Human Services. www.alzheimers.gov.
2. Tang, X., Qin, Y., Wu, J., Zhang, M., Zhu, W., & Miller, M. I. 2016. Shape and diffusion tensor imaging based integrative analysis of the hippocampus and the amygdala in Alzheimer's disease.Magnetic resonance imaging.34(8):1087-1099.
3. Yeh F, Verstynen TD, Wang Y, Fernández-Miranda JC, Tseng WI. 2013. Deterministic diffusion fiber tracking improved by quantitative anisotropy. PLoS One. 8(11): e80713.
Figures