5189
Imaging low grade inflammation in post-traumatic osteoarthritis1New York University, New York, NY, United States, 2University of Western Ontario, London, ON, Canada
Synopsis
Chronic inflammation has been identified as a major driver of joint degradation after injury. MRI is very limited to assess chronic inflammation with high specificity. Here we propose a novel contrast agent that targets the interaction of hyaluronan with RAMM cell receptors that represent an important signaling pathway for chronic inflammation. We validated the contrast agent with optical imaging and showed its value as an MRI contrast agent.
Background
Post-traumatic osteoarthritis (PTOA) is a common and serious complication after cruciate ligament (ACL) rupture.1,2 One of the driving factors in the progression to PTOA is perpetuation of inflammatory response to injury into a low-grade chronic inflammation.3-7 MRI is very limited in the assessment of low-grade inflammation in vivo. Contrast-enhanced MRI can measure inflammatory vasodilation but lacks molecular specificity and ability to assess inflammation in low-perfused tissues such as ligaments and cartilage. We developed a peptide-based imaging probe that target hyaluronan (HA)-mediated inflammatory signaling through its interactions with HA and RHAMM cell receptor.8-10Methods
Contrast agents
P15-1 is a 15-mer peptide with homology to HA binding sequences of RHAMM10 that reduces inflammation in IL1β-stimulated chondrocytes. Based on its HA-binding capability and the increased levels of HA and HA-receptors in inflamed joint tissues, we anticipated that P15-1 will bind to the sites of active inflammation. We have developed two versions of the contrast agent. First, we conjugated a Cy5.5 fluorophore to the amino-terminal group of P15-1 for near infrared (NIR) imaging and histology validation. Second, we conjugated P15-1 to a Gd-DOTA chelating complex. In both cases, same spacer was used to increase distance between peptide and conjugation site.
Animal model
We induced OA in mature male Sprague-Dawley rats (14-16 weeks of age)11 by surgical transection of the anterior cruciate ligament (ACLT).12 Eight weeks after surgery, the rats generate moderate PTOA that was confirmed by histology.
Validation studies with optical imaging
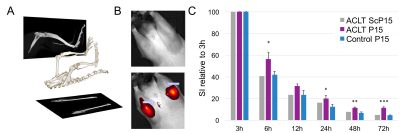
Eight weeks after surgery, rats were intraarticularly injected with 10μg/50μl of Cy5.5-P15-1 tracer using an ultrasound guided injection setup (n=4 ACLT, n=4 Sham, n=4 control). To reduce self-fluorescence on the 680 nm band animals were on alfalfa-free diet for two weeks before imaging. Rats were positioned in the IVIS scanner using a custom designed device that holds the knee in flexed with 20 degrees of internal rotation allowing for a frontal view of the tibiofemoral space (Fig.1). NIR imaging was acquired before injection, 5 minutes after injection and 3, 6, 12, 24, 48 and 72 h after injection. Tissue localization of the NIR-tracers in the joint was analyzed by fluorescence microscopy in cryosections using the tape method,13 that preserves fluorophore functionality.
Validation studies with MRI
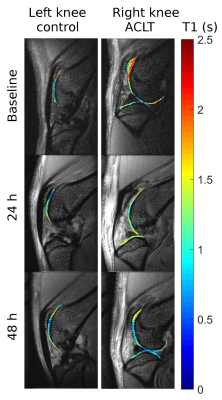
We used MRI to provide topographical information of the contrast agent distribution. We first calculated the change in relaxation rate of the contrast agent with Gd concentration (0 to 2mM) to determine an optimal dose. Then we injected both limbs of three rats intraarticularly with a combination of DOTA-Gd-P15-1 (1mM, half concentration used in humans) and Cy5.5-P15-1 (0.1mM). MRI protocol included an inversion recovery turbo-spin echo (IR-TSE) sequence (resolution=100×100×750 µm3, TE/TR=12.5/5343 ms, turbo-spin factor=5, echo spacing=6.25 s, inversion times(TI)=0.05,0.1,0.15,0.2,0.3,0.4,0.5,0.75,1,1.5,2,3,4,5 s, 8 slices, acquisition time=35 min/knee). All rats underwent bilateral knee MRI 24 h before injection, and 5, 24 and 48 h after injection on a Bruker 7T magnet using a 4-element receive surface coil. After the last imaging session, rats were sacrificed and limbs underwent fluorescence microscopy. T1 maps were calculated from the IR-TSE images and cartilage was segmented to calculate the average T1 value.
Results
Validation studies with optical imaging
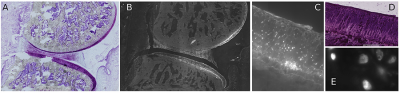
Our data shows that accumulation in ACLT limbs is higher than in control (Fig. 1) probing feasibility of the approach to track low-grade inflammation. ACLT joints showed increased retention of the contrast agent from 12 to 72 h after injection. Fluorescence microscopy showed accumulation on menisci and more predominantly in articular cartilage (Fig. 2). Interestingly, contrast agent accumulated primarily into chondrocytes, specially the ones in the deep cartilage layer. One limitation of NIR imaging is that it provides an overall measurement of contrast agent in the joint, including the fraction that is being eluted from the joint (Fig. 3).
Validation studies with MRI imaging
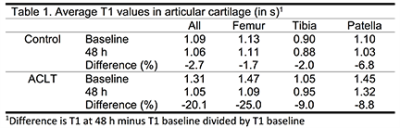
The relaxation rate (r1) of DOTA-Gd-P15-1 contrast agent was 2.82 mM-1s-1. T1 on an ACLT knee changed over time showing a clear decrease in T1 in the femoral trochlea and the patella (Fig 3). Average T1 values were increased in ACLT compared to control limbs. Over 48 hours T1 dropped in ACLT knee between -10% to -25% (-18% globally, Table 1) while it did not change in control groups (-2.7% globally). Contrast agent also accumulated in menisci and fat pad.
Conclusion
P15-1 represents a promising imaging agent for chronic low-grade inflammation in PTOA, thus with potential to provide new insight into the pathophysiology of OA that is difficult to assess otherwise. Even more, the potential anti-inflammatory effect of this peptide make it as a candidate for theranostic value that we will explore in future work.Acknowledgements
We thank the NYUSoM Experimental Pathology Research Laboratory and the Preclinical Imaging Core for advice and technical support. This research was supported by the NIAMS of the NIH under award numbers R21AR066897 and RO1AR067789.References
- Lohmander LS, Roos H. Knee ligament injury, surgery and osteoarthrosis: truth or consequences? Acta Orthop Scand. 1994;65(1):605-609.
- Lohmander LS, Englund PM, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. The American journal of sports medicine. 2007;35(10):1756-1769.
- Olson SA, Horne P, Furman B, et al. The role of cytokines in posttraumatic arthritis. J Am Acad Orthop Surg. 2014;22(1):29-37.
- Furman BD, Mangiapani DS, Zeitler E, et al. Targeting pro-inflammatory cytokines following joint injury: acute intra-articular inhibition of interleukin-1 following knee injury prevents post-traumatic arthritis. Arthritis research & therapy. 2014;16(3):R134.
- Lieberthal J, Sambamurthy N, Scanzello CR. Inflammation in joint injury and post-traumatic osteoarthritis. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2015;23(11):1825-1834.
- Harkey MS, Luc BA, Golightly YM, et al. Osteoarthritis-related biomarkers following anterior cruciate ligament injury and reconstruction: a systematic review. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2015;23(1):1-12.
- Haseeb A, Haqqi TM. Immunopathogenesis of osteoarthritis. Clinical immunology (Orlando, Fla). 2013;146(3):185-196.
- Petrey AC, de la Motte CA. Hyaluronan, a crucial regulator of inflammation. Frontiers in immunology. 2014;5:101.
- Yang C, Cao M, Liu H, et al. The high and low molecular weight forms of hyaluronan have distinct effects on CD44 clustering. The Journal of biological chemistry. 2012;287(51):43094-43107.
- Tolg C, Hamilton SR, Zalinska E, et al. A RHAMM mimetic peptide blocks hyaluronan signaling and reduces inflammation and fibrogenesis in excisional skin wounds. The American journal of pathology. 2012;181(4):1250-1270.
- Boyan BD, Tosi LL, Coutts RD, et al. Addressing the gaps: sex differences in osteoarthritis of the knee. Biology of sex differences. 2013;4(1):4.
- Ramme AJ, Lendhey M, Raya JG, Kirsch T, Kennedy OD. A novel rat model for subchondral microdamage in acute knee injury: a potential mechanism in post-traumatic osteoarthritis. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2016.
- Dyment NA, Jiang X, Chen L, et al. High-Throughput, Multi-Image Cryohistology of Mineralized Tissues. J Vis Exp. 2016;115.
Figures