5181
Feasibility of Quantitative Ultrashort Echo Time Magnetization Transfer (UTE-MT) Modeling Based on Undersampled Acquisitions and ESPIRiT Reconstruction1Shenzhen Institutes of Advanced Technology, Chinese Academic of Sciences, Shenzhen, China, 2Department of Radiology, University of California, San Diego, San Diego, CA, United States, 3Radiology Service, VA San Diego Healthcare System, San Diego, CA, United States
Synopsis
Recently the ultrashort echo time magnetization transfer (UTE-MT) technique with two-pool modeling has shown promise as a clinically compatible quantitative technique which is resistant to the magic angle effect. However, the UTE-MT quantitative technique requires multiple acquisitions, which result in suboptimal acquisition times. The purpose of this study is to assess the feasibility of undersampled acquisitions for quantitative UTE-MT modeling applications.
Introduction
Magnetization transfer (MT) imaging has been used for indirect assessment of macromolecules in biological tissues1. MT modeling provides abundant quantitative information, such as the fractions, relaxation times and exchange rates of different proton pools, and has shown promise as a clinically compatible quantitative technique. Furthermore, MT modeling parameters have been shown to be magic angle insensitive2. However, acquisition of multiple MT datasets, including different saturation powers and frequency offsets, are required for MT modeling. Despite higher ratios of acquisition spokes per saturation pulse3, imaging times remain long, particularly when volumetric imaging is used. The purpose of this study is to assess the feasibility of undersampled acquisitions for quantitative UTE-MT modeling applications.Material and Methods
Specimens: This anonymized cadaveric study included 8 knee samples (4 females, 4 males; mean age 57 years-old, range 24-88 years-old) was approved by our Institutional Review Board. Specimens had undergone a single freeze-thaw cycle prior to scanning.
Protocol: Imaging was performed on a 3T clinical MRI scanner (GE MR750). An eight-channel knee coil was used for signal excitation and reception. The 3D UTE-MT-Cones sequence employed a MT preparation followed by multiple 3D UTE Cones interleaves to accelerate data acquisition3. The MT preparation consists of a Fermi shaped RF pulse (duration = 8 ms, bandwidth = 160 Hz) followed by a gradient crusher. The 3D UTE-MT imaging parameters include: TR = 102.3 ms, TE = 32 μs, FA = 7°, BW = ± 83.3 kHz, FOV = 15 cm, reconstruction matrix = 256 × 256, slice thickness = 2 mm, slice number = 60; nine interleaves per MT preparation pulse, three MT powers (500°, 1000° and 1500°) and five MT frequency offsets (2, 5, 10, 20 and 50 kHz), with a total of 15 different MT datasets. Undersampled acquisitions with acceleration factors (AF) equal to 1/2/4/6/8 were separately acquired, with the total scan time of ~60/30/15/10/7.5 min. T1 was measured with the 3D UTE-Cones fully-sampled acquisition with the same plane, spatial resolution and other imaging parameters including: TRs = 20/40/60/80/100 ms, TE = 32 μs, FA = 45°.
Image Reconstruction and Quantitative Measurement: Conventional regridding and the recently described l1-ESPIRiT reconstruction were performed on original k-space datasets using BART (Berkley Advanced Reconstruction Toolbox)4, 5. The quantitative measurement algorithm was written in MATLAB (MathWorks, 2016b, Natick, MA, USA) and was executed offline on the images obtained by the above reconstruction methods. A Levenberg-Marquardt algorithm was employed for the non-linear least squares fitting in MT modeling, as well as T1 fitting. Two-pool UTE-Cones-MT modeling and parameter mapping were performed on the datasets using previously described methods2, 3. Regions of interest (ROIs) were drawn using the fully sampled UTE-T1 images with an average of 100 pixels per ROI. The same ROI was executed among undersampled datasets for comparison. Mean and standard deviation of macromolecular proton fraction, relaxation time, exchange rates and water longitudinal relaxation were measured in various joint tissues including cartilage, meniscus, patellar tendon and the posterior cruciate ligament (PCL).
Results
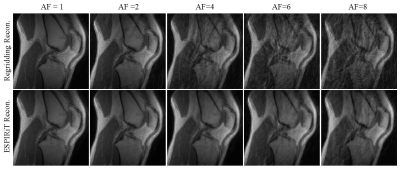
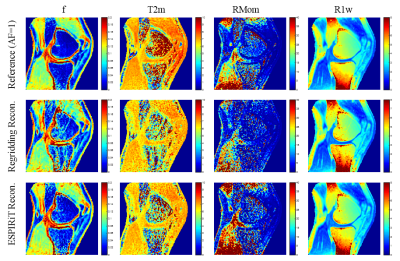
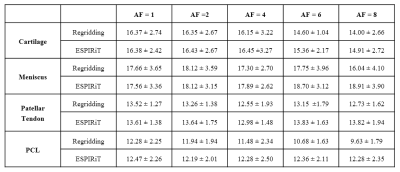
Figure 1 shows the regridding and ESPIRiT reconstruction results using different acceleration factors. The ESPIRiT reconstruction algorithm provides noticeably less artifacts while maintaining image quality, even with a quarter of the acquisition data (AF=4). Corresponding UTE-MT modeling fitting curves in Figure 2 show obvious variation in values from acceleration factors of 6 and 8 using regridding. However, all UTE-MT modeling fitting curves are similar (fraction ~13.5%, STD = 0.25%) with excellent fit using ESPIRiT. Quantitative maps of MT modeling were calculated between two reconstruction methods using the AF=4 dataset. Figure 3 shows quantitative MT maps from fully sampled acquisition data as reference in the first row, and the other rows in Figure 3 compare quantitative MT maps from these two reconstruction methods using AF=4 datasets. Comparison of macromolecular fractions generated from the two reconstruction methods with different acceleration factors are listed in Table 1.Conclusions
Undersampled acquisitions with ESPIRiT reconstruction can dramatically decrease streaking artifacts even with four-fold acceleration, and provide similar quantitative maps as fully-sampled acquisitions. ROI-based analyses appear more resistant image artifacts compared with quantitative pixel maps.Acknowledgements
The authors gratefully acknowledge grant support from NIH (1R01 AR062581 and 1R01 AR068987), VA Clinical Science Research and Development Service (Merit Award I01CX001388)), National Key Research and Developed Program of China (2016YFC0105102), National Science Foundation of Guangdong (2014A030312006), and GE Healthcare.References
1. Wolff SD, Balaban RS. Magnetization transfer contrast (MTC) and tissue water proton relaxation in vivo. Magn Reson Med 1989; 10:135-144.
2. Ma YJ, Shao H, Du J, et al. Ultrashort echo time magnetization transfer (UTE-MT) imaging and modeling: magic angle independent biomarkers of tissue properties. NMR Biomed. 2016; 29(11):1546-1552.
3. Ma YJ, Chang EY, Carl M, et al. Quantitative Magnetization Transfer Ultrashort Echo Time Imaging Using a Time-Efficient 3D Multispoke Cones Sequence. Magn Reson Med. 2017 (in print).
4. Uecker M, Lai P, Murphy MJ, et al. ESPIRiT—An Eigenvalue Approach to Autocalibrating Parallel MRI: Where SENSE Meets GRAPPA. Magn Reson Med. 2014:71:990-1001.
5. Uecker, Martin, et al. "Berkeley advanced reconstruction toolbox." Proc. Intl. Soc. Mag. Reson. Med. 2015; Vol. 23
Figures