5176
Protocol for in vivo acquisition and fully-automated segmentation of the anterior cruciate ligament1Biomedical Imaging Center, Pontificia Universidad Católica de Chile, Santiago, Chile, 2Department of Electrical Engineering, Pontificia Universidad Católica de Chile, Santiago, Chile, 3Department of Orthopedics and Traumatology, School of Medicine, Pontificia Universidad Católica de Chile, Santiago, Chile, 4Department of Radiology, School of Medicine, Pontificia Universidad Católica de Chile, Santiago, Chile, 5Institute for Biological and Medical Engineering, Pontificia Universidad Católica de Chile, Santiago, Chile
Synopsis
In this preclinical study we propose a protocol for rapid 3D imaging and fully automated segmentation to create a standardized healthy ACL image database. The segmentation problem of the ACL is particularly challenging due to its poor contrast. Our protocol demonstrated promising fully-automated segmentation of the ACL. Thus, allowing us to have a 3D computational model of the ACL. Ongoing experimentation explores dynamic imaging of the ACL in motions of flexion-extension. Such work will improve understanding of in vivo knee mechanics with potential to inform treatment of different injuries related to the ACL.
Introduction
Anterior cruciate ligament (ACL) injuries are among the most common knee related injury. Most patients that suffered from ACL injuries will demonstrate osteoarthritis in their lifetime1, exhibiting faster on patients receiving surgical treatment. Currently, surgical ACL reconstruction techniques rely on physiologically limited models: static in vivo MR images or dynamic simulations that lack the utility of in vivo images. Furthermore, a standardized database does not exist which is essential to understand the geometry at different levels of flexion of the healthy ACL. At the same this database will improve atlas based segmentation. In this preclinical study, we propose a protocol for rapid 3D imaging and fully-automated segmentation. Due to the above, the aim is to create a standardized healthy ACL image database. Ongoing experimentation explores dynamic imaging of the ACL in flexion.Methods
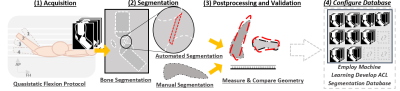
(1) We scanned 14 volunteers in a 1.5T Phillips scanner: T2-weighted; Isotropic resolution of 0.7mm; FOV of 156x156x22mm; 3D TSE Cartesian; Total time of 4:40s.
(2) We manually segmented out the tibia and femur. Afterwards, using Horos software, we manually segmented the ACL axial to the FOV. For fully automated (FA) segmentation, we used an Atlas based sparse reconstruction classifier2,3,6,7 trained with manual segmentations.
(3) To test the utility of the segmented data, we measured the length, medial area, and volume of each ACL (these were chosen for simplicity). Using DICE spatial correlation and Cohen’s kappa coefficient, we compared the manual and automated volumes.
(4) With our measurements, we created the database.
The study includes 13 knees in flexion and 12 knees in extension. We omitted one knee (flexion and extension) because of an acquisition problem and one knee in extension because its ACL was too fatty and was an outlier in our database.
Results
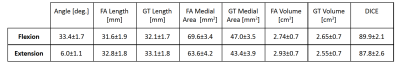
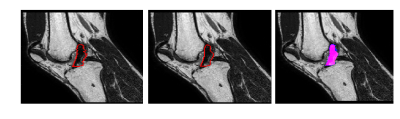
Table 1 shows the results obtained by the FA segmentation for the chosen measures. It, shows a good correlation against manual segmentation, which was considered the ground truth (GT). Figure 2 shows the segmentation of a single slice in the sagittal plane. The average computation time for each segmentation was 23 minutes in a standard laptop.
Discussion
The manual geometries resemble those reported in existing ACL studies4,5. With the exception of the medial area, the FA volumes and lengths fall within and close to the manual segmentations. The FA medial cross-sectional area was consistently larger than the manual. This is due to the inclusion of intersecting tissue in the medial zone of the ACL. Comparing DICE values with reported literature4, the FA methods correlates well against the manually segmented volumes. Using our DICE observation, the flexion and extension measurements have a Cohen’s kappa coefficient of 0.88 and 0.85 respectively. Overall, the results of the FA method are promising and the FA segmentation will improve with a larger database. A reliable FA segmentation will expand the utility of our protocol by eliminating manual segmentation; saving both time and expertise during ACL database development.Conclusions
Our protocol demonstrated promising anatomical segmentation of the normal ACL in preliminary experiments. In the future, a bigger and more diverse database will allow for better results. Continued experiments will use the principles of joint compressed sensing and parallel imaging for dynamic visualization of the ACL during flexion or torsion. Such work will improve understanding of in vivo knee mechanics with potential to inform treatment of ACL related injury.Acknowledgements
The first author was supported by the Whitaker International Program and the study received funding from Anillo ACT1416.References
1. Ajuied A, Wong F, Smith C, et al. Anterior cruciate ligament injury and radiologic progression of knee osteoarthritis: a systematic review and meta-analysis. The American Journal of Sports Medicine. 2014, p. 2242-52.
2. Tonga T, Wolza R, et al. Segmentation of MR images via Discriminative Dictionary Learning and Sparse Coding: Application to Hippocampus Labeling. Neuroimage. 2013 Aug 1;76:11-23.
3. Coupé P, Manjón J, et al. Patch-based segmentation using expert priors: Application to hippocampus and ventricle segmentation. . Neuroimage. 2011 940-954.
4. Lee H, Hong H, and Kim J, Segmentation of anterior cruciate ligament in knee MR images using graph cuts with patient-specific shape constraints and label re fi nement, Comput. Biol. Med., vol. 55, pp. 1–10, 2014.
5. Kupczik F , Eduardo M. , Schiavon G, Sbrissia B, Caldonazzo R, and Valério R, ACL ideal graft : MRI correlation between ACL and humstrings , PT and QT, Rev. Bras. Ortop., vol. 48, no. 5, pp. 441–447, 2013.
6. Mairal J, Bach F, Ponce J, Sapiro G. Online Learning for Matrix Factorization and Sparse Coding. Journal of Machine Learning Research, volume 11, pages 19-60. 2010.
7. Mairal J, Bach F, Ponce J, Sapiro G. Online Dictionary Learning for Sparse Coding. International Conference on Machine Learning, Montreal, Canada, 2009.
Figures