5171
MRI, CT, and histomorphometry co-registration studies: analysis of murine tibia morphology before and after decalcification processMarilena Tauro1, William Dominguez-Viqueira1, Jan Poleszczuk2, Conor C Lynch1, Gary Martinez1, and Mikalai M Budzevich1
1Moffitt Cancer Center, Tampa, FL, United States, 2Polish Academy of Sciences, Warsaw, Poland
Synopsis
In this work we evaluate the relative merits of MRI and CT to “gold standard” histopathology assessment, with regard to bone tumor metastases. We also investigate the profound effect that decalcification process has on tumor/bone morphology. MRI shows differences in tumor shape before and after decalcifications, however the differences in total tumor volume were smaller than 5 percent. Co-registered microCT images and MR images exhibit displacement of bone-matrix after decalcification. The question of co-registration between all three modalities remains open, however we believe our algorithm minimizes registration errors.
Introduction
Skeletal disease is common in patients with malignancies such as multiple myeloma and bone metastatic cancer1,2. In bone, cancer cells interact with the surrounding stroma to generate lesions that are osteolytic (bone destructive) or osteoblastic (bone forming) or, in most cases, a mixture of both processes3. The site of bone colonization/metastasis can vary but typically, they manifest in the axial skeleton, in the metabolically active metasphyseal regions of trabecular bone. Understanding the anatomical habitat of early lesions can reveal clues as to how cancer cells disseminate to bone and establish active disease. Co-registration of imaging modality data with histological decalcified tissue sections can address this underexplored issue. Computed tomography (CT) provides high resolution of cortical and trabecular bone (up to 3 microns). Magnetic Resonance Imaging (MRI) is complementary to CT in that it is sensitive to soft tissue and in particular, the tumor, bone marrow and skeletal metastasis before cortical destruction occurs4,5. This can be used to assess treatment response, as well as size and number of osseous metastases over time. In order to evaluate the relative merits of each modality with regard to bone tumor metastases, we seek to relate MRI and CT to “gold standard” histopathology assessment. We also investigate the profound effect that decalcification has on tumor/bone morphology.Methods
All experiments were performed with University of South Florida IACUC approval. Luciferase expressing multiple myeloma cancer cells were injected (1×105 in 10 μl volume) into the tibia of an anesthetized immunocompromized mouse while contralateral limbs received sham injections of saline. Tumor growth was monitored overtime using bioluminescence imaging system IVIS 200 (Caliper Life Science, Hopkinton, MA) and after three weeks, the animals were euthanized. The tumor bearing and control limbs were carefully excised and soft tissue removed. After 24 h fixation in formalin, all bones are transferred in 70% ethanol solution prior to imaging procedures. Each sample was imaged with 3 modalities: (MRI, microCT Inveon and Scanco). MRI was performed before and after decalcification on a 7T horizontal magnet (Agilent-Technologies) with Bruker electronics (BioSpec AV3HD), using a two-element 1H cryoprobe. A turboRARE sequence with TR/TE of 4066/55ms, 4 averages and 16 rare-factor was used to acquire 25 slices of 250 mm thickness and 67×67 mm in-plane resolution. MicroCT scans were performed prior to decalcification on Inveon™ preclinical scanner (Siemens medical, Knoxville, Tennessee) as well as on Scanco μCT 35 (Scanco Medical, Fabrikweg, Switzerland) using similar scan parameters (80 kVp and 300 µA for Inveon, versus 70 kVp and 400 μA). Bones were decalcified in 14% EDTA at pH 7.4 for two weeks, paraffin embedded and sectioned for histologic analysis. Tissue sections of 5μm thickness, were sampled at sequential points throughout the specimen. The sections were processed (rehydration through ethanol series 100% to 50% and then H2O) and H&E stained. Images for the same bone were co-registered to 3D MRI, 3D CT, and histological slices using MATLAB generated code and Inveon™ multimodal 3D visualization software. Tumor volumes were segmented in MR images and quantitatively compared using Dice coefficients. The spatial distribution of tumor in bone (using MRI) was compared to the trabecular bone distribution in microCT images.Results
MRI shows differences in tumor shape before and after decalcification (Fig.1a, b), however the differences in total tumor volume was smaller than 5% (Fig. 2a). Co-registered microCT images and MR images exhibit displacement of bone-matrix after decalcification. The red contour on Fig.3d depicted initial boarders of tumor and tumor’s boarders after decalcification Fig.3f. Tumors borders in decalcified bone have shown the displacement of trabecular bone matrix by soft tumor masses. Co-registration of histological slices with microCT images (see Fig.4) resulted in Dice coefficients in the range of 60%-90%.Discussion
We believe the tumor shape and volume is partially affected, as shown in MR images, due to changes in the bone matrix and infiltration of protons during the decalcification process. The sham and the tumor bearing samples were both fixed, and hence, the potential effect of fixing on their comparative behavior to detect abnormalities should be minimized. The question of co-registration between all three modalities remains open, however we believe our algorithm minimizes registration errors.Conclusion
The bone decalcification procedure does not significantly affect tumor volume but does change a tumor/trabecular bone spatial distribution. The information from all modalities can be combined as one-to-one structural correspondence, but it requires introduction of normalization factor. Moving forward, we believe this multi-modal co-registration approach can be used to reveal important insights into the exact anatomical niches that are preferentially colonized by cancer cells.Acknowledgements
No acknowledgement found.References
- Yu H.H., Tsai Y.Y., Hoffe S.E. Overview of diagnosis and management of metastatic disease to bone. Cancer Control. 2012 Apr; 19(2):84-91.
- Zekri J., Marples M., Taylor D., Kandukurti K., McParland L., Brown J.E. Complications of bone metastases from malignant melanoma. Journal of Bone Oncology. 2017; 8:13-17.
- Bussard, Karen M., Carol V. Gay, and Andrea M. Mastro. “The Bone Microenvironment in Metastasis; What Is Special about Bone?” Cancer and Metastasis Reviews 27, no. 1 (March 1, 2008): 41–55.
- O’Sullivan, G. J., Carty, F. L., & Cronin, C. G. (2015). Imaging of bone metastasis: An update. World Journal of Radiology, 7(8), 202–211.
- Bäuerle T., Semmler W. Imaging response to systemic therapy for bone metastases. Eur Radiol. 2009 Oct; 19(10):2495-507.
Figures
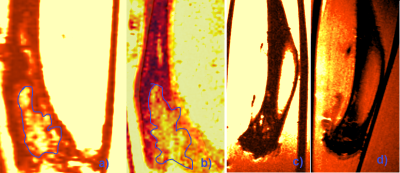
Figure 1. Coronal ex-vivo MR image of tumor bearing bone before (a)
and after (b) decalcification, MR image of non-tumor bearing bone before (c)
and after (d) decalcification. Blue line depicted tumor in bone.
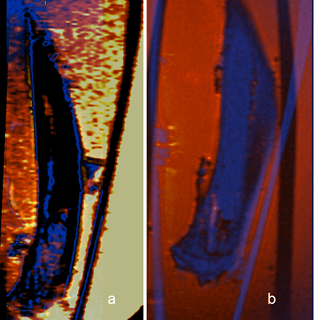
Figure 2. Coronal MR images co-registration of ex-vivo bones. Co-registered
MR images of tumor bearing bone before (a) and after (b) decalcification. The
blue scheme correspond calcified bone image, the red scheme represents the
de-calcified bone image.
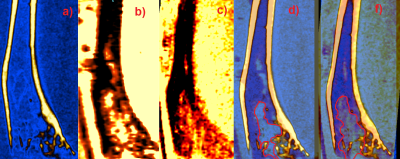
Figure 3. a) MicroCT image of tumor-bearing bone, b) MR image of
tumor-bearing bone before de-calcification, c) MR image of tumor-bearing bone
after de-calcification, d) MRI and microCT
co-registration of ex-vivo bones before, and f) after decalcification. The red contours
on d) and f) shows tumor boundaries on MR image.
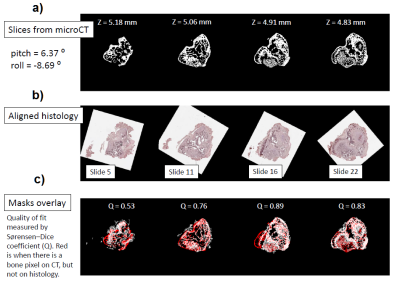
Figure 4. Co-registration of microCT images with corresponding histological
slices. a) microCT
images of the slices at 4 different z positions. b) Aligned histological slices
corresponding to microCT images on a. c) Image fusion of the co-registered microCT and histological images.