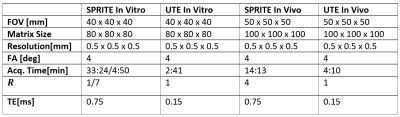
5148
Rapid enhanced SPI imaging using inductively coupled local coils for metal artifact reduction1Internal Medicine 2, Ulm University Medical Center, Ulm, Germany, 2Department of Radiology and Neuroradiology, University Medical Center Kiel, Kiel, Germany, 3Department of Radiology, University Medical Center Freiburg, Freiburg, Germany
Synopsis
As a pure phase encoding technique, Single Point Imaging (SPI) has a great potential to reduce metal-induced artifacts, at the cost of long acquisition times normally not suited for clinical use. In this contribution, we present an approach combining SPI with reduced field-of-view imaging and SPARSE-SENSE reconstruction techniques, by using inductively coupled coils (ICC) for local signal boosting and multi-element receive coils. Initial phantom and in-vivo results show reduced metal artifacts at clinically acceptable scan times with the proposed techniques.
Introduction
Metallic implants are known to cause severe susceptibility image artifacts including distortions, signal pile-up and loss. SPI imaging techniques, like SPRITE1, have the potential to reduce the induced artifacts. The inherent drawbacks of SPI techniques result from 1) the prolonged scan times caused by the single k-space point readout after each excitation; 2) the intrinsic low SNR limiting the use of high undersampling factors; and, in case of SPRITE, 3) the high excitation bandwidth required for excitation of large field-of-views (FOV) in the presence of a high readout gradient. To solve these problems, in this contribution under-sampled SPRITE was combined with data acquisition using a small volume coil (ICC), inductively coupled to a four-element receive array thus reducing the FOV while enabling SPARSE-SENSE reconstruction.Methods
All in vitro and in vivo scans were performed with a 3T whole-body scanner (Achieva 3T, Philips Healthcare, Best, The Netherlands) , using one section of a 2 times four-element receive coil (Carotid Coil, Shanghai Medical Technologies Co., Shanghai, China), and an ICC2 with a small sensitivity range. Due to the ICCs design, the acquired signal within its sensitivity area is boosted and dominating the induced signal in the four-element receive-coil, thus reducing the FOV, while improving local SNR and maintaining the parallel imaging properties of the receive coil.
All data was acquired with the SPRITE imaging sequence and a 3D Ultra Short Echo Time (UTE) scan for image quality comparison. A 25.6µs excitation block pulse was used, to excite the probe inside the sensitivity region of the ICC evenly. The scan parameters are provided in Table 1. A combination of radial and polar undersampling was used to ensure best possible incoherent sampling properties for the SPRITE sequence. In-vitro data was obtained from a tooth with large amalgam filling embedded in agarose. In-vivo data was obtained from the interphalangeal joint of the middle finger. To mimic susceptibility effects, an additional scan was performed with a bracket and an orthodontic wire attached to the finger. Acceleration factor of R=7 for in vitro and R=4 for in vivo measurements were tested.
Reconstruction of the undersampled SPRITE data was performed applying SPARSE-SENSE3 reconstruction with total variation sparsity transformation. K-space sampling density was estimated by Voronoi tessellation4,5 for undersampled data6. All reconstructions were performed with an in-house build reconstruction framework, implemented in Matlab (Matlab2016b,The MathWorks, Natick, Massachusetts, USA).
Results and Discussion
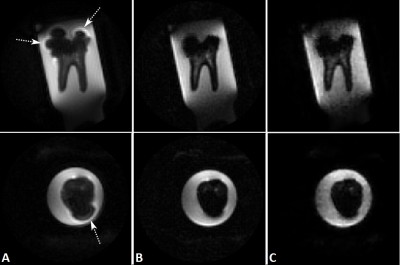
Figure 1. shows the UTE (A), the fully sampled (B) and 7-fold undersampled SPRITE (C) images, for two orthogonal orientations. In comparison to the conventional UTE sequence, a clear reduction of the metal artifact can be appreciated in the SPRITE images. Minor deterioration in the high-susceptibility region of the filling can be observed for the 7-fold undersampling.
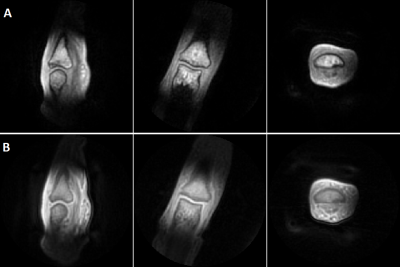
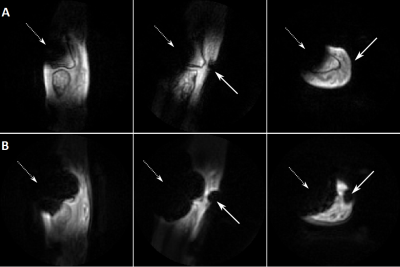
In vivo, the four-fold accelerated SPRITE image shows an acceptable SNR within the sensitivity range of the ICC (Figure 2). The sensitivity for short T2* values is higher in the UTE images (B) in comparison to the SPRITE images (A), due to the shorter echo time. In presence of metal objects, the four-fold undersampled SPRITE sequence does not show any image distortions and a clear reduction of the signal voids (Figure 3, A) in comparison to the UTE sequence (B).
Conclusion
The presented approach has been successfully applied to reduce metal artifacts in human SPRITE imaging for a small FOV at acceptable scan times and SNR. Future applications could be intra oral metal artifact reduction. The use of Quasi Random sampling6 would further increase the incoherent sampling properties and therefore allow higher undersampling factors for in vivo applications.Acknowledgements
JBH: Support by the DFG (HO-4604/2-1) is acknowledged.References
[1] Mastikhin I V, Balcom B J, Prado P J, et al. SPRITE MRI with Prepared Magnetization and Centric k-space Sampling. Journal of Magnetic Resonance. 1999;136;159-168.
[2] Ludwig U, Eisenbeiss A K , and Scheifele C. Dental MRI using wirless intraoral coils. J. Scientific Reports. 2016;6:23301.
[3] Lustig M, Donoho D, Santos J, et al. Compressed sensing MRI. IEEE Signal Process. Mag. 2008; 25(2):72-82.
[4] Voronoi G. Nouvelles applications des paramètres continus à la théorie de formes quardratiques. Journal für Reine und Angewandte Mathematik 1908;134:198-287.
[5] Rasche V, Proska R, Bornert P, et al. Resampling of data between arbitrary grids using convolution interpolation. IEEE Trans. Med. Imaging. 1999;18:385-392.
[6] Speidel T, Paul J, Wundrak S, et al. Quasi-Random Single-Point Imaging using Low-Discrepancy k-Space Sampling. IEEE Trans. Med. Imaging. 2017; doi: 10.1109/TMI.2017.2760919.
Figures