5138
The interplay of MRS measured skeletal muscle acetylcarnitine, mitochondrial function and glucose availabilityAnne Tonson1, Robert W Wiseman1,2, Ronald A Meyer1, Taylor Ann Callahan2, Ashley Lang2, and Jill M Slade2
1Physiology, Michigan State University, East Lansing, MI, United States, 2Radiology, Michigan State University, East Lansing, MI, United States
Synopsis
Recently resting muscle acetylcarnitine content (AC) has been proposed as a marker for peripheral insulin resistance. However, muscle oxidative capacity and glucose availability may largely contribute to interindividual AC fluctuations independent of peripheral insulin sensitivity. In this study we monitored resting muscle AC in healthy subjects using 1H MRS in response to carbohydrate ingestion and examined the relationship of fasting muscle AC to muscle oxidative capacity measured by 31P MRS. Our results show a strong relationship between mitochondrial capacity and fasting muscle AC and also show that carbohydrate ingestion causes a rapid sharp decline in muscle AC.
Introduction
Skeletal muscle acetylcarnitine (AC) is reportedly involved in glucose homeostasis and metabolic flexibility1. Muscle AC content has a strong correlation with whole body insulin sensitivity when examined across healthy controls and diabetics2. It is widely accepted that exercise improves insulin sensitivity both acutely and chronically. Muscle AC may play a critical role in the improvement in insulin sensitivity with exercise response and adaptations. While it is well known that AC formation plays a buffering role in the presence of excess acetylCoA formation3, other mechanisms involved in AC accumulation or metabolism are poorly understood. Prior muscle biopsy data suggest that carbohydrate ingestion induces metabolism of AC4, however the mechanism and onset of AC metabolism with carbohydrate ingestion remains virtually unexplored. In this context proton spectroscopy (1H MRS) may provide a powerful tool to better understand the mechanisms underlying the relationship between muscle AC and peripheral glucose management.Purpose
The purpose of the current study was to examine the relationship between physical activity, in vivo mitochondrial function and muscle AC in healthy subjects using 1H and phosphorus (31P) MRS. Further, this study evaluated the influence and time course of muscle AC and blood glucose following an overnight fast and consumption of a carbohydrate beverage.Methods
Muscle AC content was measured after an overnight fast and then subsequently following consumption of 50g of simple carbohydrate (Gatorade). Blood glucose (BG) and muscle AC was measured every 15-30 minutes over a 120-min period following the beverage. Muscle AC was measured in the medial gastrocnemius using localized proton magnetic resonance spectroscopy (MRS) at 3T (PRESS, TR/TE=4000/350ms, voxel=30ml, time=5.30min); AC is expressed relative to creatine (AC/Cr). BG was measured from capillary samples obtained from the fingertip. Eleven healthy participants (7 males) were tested (age=28±3 years old (±SE), BMI=25±1). On a separate day, plantar flexor muscle oxidative capacity was measured using Phosphorus MRS acquired using a 10-cm diameter surface coil. Physical activity (PA) was measured by accelerometry for 7 days.Results
Following an overnight fast of 12±2 hours, AC ranged from 0.85 to 0.16 with an average of 0.39±0.08. Fasting AC was correlated with PA (r=0.733, p=0.010) and oxidative capacity (r=-0.813, p=0.002; Fig 1). Following carbohydrate ingestion, BG significantly increased with a peak at 30min and returned to basal levels by 90min (p≤0.001). Muscle AC was decreased as early as 15 minutes after carbohydrate ingestion and remained depressed 120min postprandial (p=0.005; Fig 2).Conclusion
In healthy subjects, daily physical activity and oxidative capacity are highly correlated to fasting muscle AC. Aerobic exercise interventions may therefore provide a means for increasing muscle AC. Given that insulin sensitivity is highly related to muscle AC2, these results may suggest that muscle AC contributes to exercise induced adaptations of insulin sensitivity. These results also show that systemic glucose availability and insulin-stimulated glucose uptake influence muscle AC content. The underlying mechanisms explaining the metabolism of AC with carbohydrate feeding and persistent reduction following feeding are unclear. In general, the results demonstrate that 1H MRS can clearly be utilized to monitor in vivo AC metabolism.Acknowledgements
Supported by NIH DK095210 & NIH DK106292References
- Muoio, D. C. A. M. et al. Muscle-Specific Deletion of Carnitine Acetyltransferase Compromises Glucose Tolerance and Metabolic Flexibility. Cell Metabolism 15, 764–777 (2012).
- Lindeboom, L. et al. Long–echo time MR spectroscopy for skeletal muscle acetylcarnitine detection. Journal of Clinical Investigation 124, 4915–4925 (2014)
- White, L. J. et al. Accumulation of Acetyl Groups Following Cycling: A 1 H-MR Spectroscopy Study. International Journal of Sports Medicine 27, 100–104 (2005).
- Watt, M.J., et al. Carbohydrate ingestion reduces skeletal muscle acetylcarnitine availability but has no effect on substrate phosphorylation at the onset of exercise in man. The Journal of Physiology 544.3: 949-956 (2002).
Figures
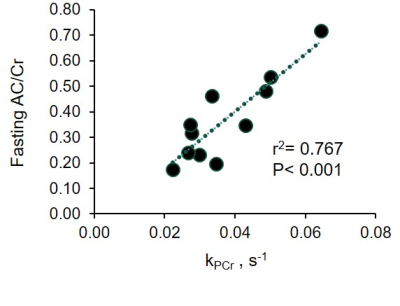
Fig 1.The relationship
between plantar flexor muscle acetlycarnitine (AC/Cr) measured with proton MRS and muscle
oxidative capacity measured with 31P MRS.
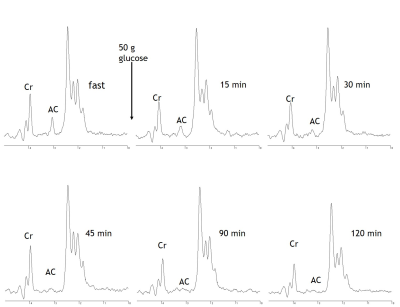
Fig 2. MRS spectra acquired from the medial gastrocnemius from a representative subject are shown. Spectra show fasting acetylcarnitine (AC) content and the decline in muscle AC following carbohydrate ingestion. Cr = creatine.