5132
Multi-Nuclei Functional Imaging of the Skeletal Muscle during Synchronized Electrical Stimulation: Interleaving 1H Velocity Mapping with 31P Spectroscopy1Radiological Physics, University Hospital Basel, Basel, Switzerland, 2Department of Biomedical Engineering, University of Basel, Basel, Switzerland, 3Neuro- and Developmental Pediatrics, University Children's Hospital Basel, Basel, Switzerland
Synopsis
In this work, we present a novel multi-nuclei interleaved acquisition sequence that acquires three-directional phase contrast images and phosphorus spectra during muscle exercise. The sequence is combined with electrical muscle stimulation to achieve a comprehensive investigation method of the functionality of the skeletal muscle.
Introduction
The active assessment of muscle functionality by MR can be divided into two broad categories: dynamic 1H imaging, giving information on the kinematics and mechanical properties of the muscle, and 31P spectroscopy, to obtain information about muscle metabolism and energetics.
As an additional tool, for its good objectivity and repeatability, electrical muscle stimulation (EMS) has been successfully used for muscle exercise during MR acquisition1,2. Although both examinations are important for a complete assessment of the muscle condition, due to the very different technical requirements, standard examination protocols only include one of them.
In order to optimize the acquisition and to have a complete picture of the muscle status and functionality during exercise, we hereby propose an interleaved approach (similar to Lopez et al.3), where 1H and 31P acquisitions are alternated without the need of hardware modifications. To this end, we present a novel acquisition sequence that acquires 1H velocity images during EMS-induced contractions and uses the relaxation phase of the contraction to acquire a metabolic 31P spectrum, thus obtaining both kinematic and metabolic information during a single exercise session. This way, we can optimize the scan time, reduce muscle fatigue and consequently reduce the associated confounding factors.
Methods
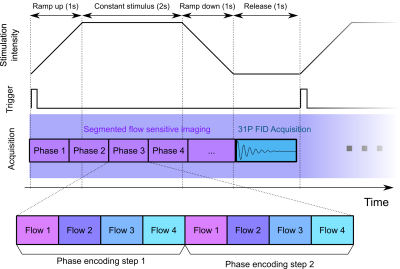
An interleaved multi-nuclei (1H imaging and 31P spectroscopy) MR acquisition sequence was developed and tested in vivo at 3T field strength. The imaging part consisted of a cine triggered, segmented, single-slice, three-directional phase contrast flow encoding sequence with Hadamard (4-point balanced) flow encoding4. After the acquisition of every phase loop (i.e. once per contraction), a single 31P nonlocalized free induction decay (FID) was acquired. Because the triggering and the contraction was delivered by an electrical muscle stimulator, the timing was set appropriately in order to acquire the phase contrast imaging during the contraction and release of the muscle, and the FID during the quiescent phase before the next stimulation (Fig. 1). This is necessary to avoid RF interferences in the phosphorus spectrum, which has a lower signal level with respect to proton imaging, due to the lower natural abundance of the element5.
The sequence was implemented on a 3T human whole-body clinical scanner equipped with a dual-tuned 1H/31P surface coil and tested on a healthy volunteer. EMS was delivered through saline-based electrodes placed on the triceps surae, with a periodic stimulation with a 5s period and maximum amplitude of 18mA. This stimulation was sufficient to elicit a visible submaximal contraction of the muscle. The phase contrast acquisition had a resolution of 2.3x2.3x5.0mm3, matrix size 128x96x1, 2 k-space lines per phase, TE/TR 6.85/10.95ms, temporal resolution 87ms, 51 dynamic phases, Venc 25cm/s. Interleaved with the phase contrast acquisition, a hard phosphorus pulse of 42° was followed by a phosphorus ADC with a bandwidth of 2000Hz and a vector size of 1024 points. The total acquisition lasted 4 minutes (48 contractions).
Results
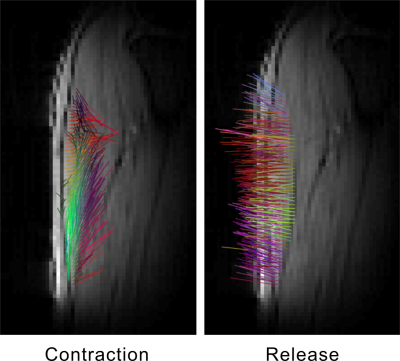
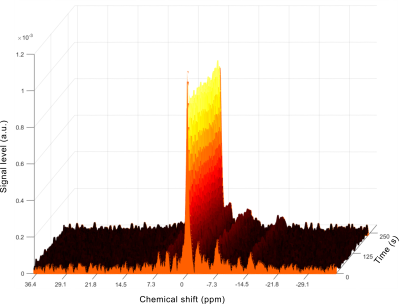
The sequence ran successfully on the scanner, acquiring a single-slice three-directional dataset during the 4-minute acquisition. The images did not present any noticeable artifact (Fig. 2) and reconstruction of the velocity vector field was possible, with correct depiction of the muscle contraction and release phase (Fig. 3). The acquired spectra were also properly processed and the principal phosphorus metabolites (Pi, PCr, ATP) were visible without appreciable artifacts (Fig. 4). Due to the low intensity of the current, no significant Pi increase was observed during the stimulation.Discussion
The implementation of an interleaved sequence able to acquire 1H images and 31P spectra in the same acquisition presented a number of technical challenges due to the characteristics of the programming framework available for a commercial scanner, which is not intended to be used for interleaved MR acquisitions.
Therefore, it required a complete manual reimplementation of the whole sequence code and offline reconstruction of the raw data. Moreover, the usage of the electrical stimulation required careful timing of the acquisition to avoid contamination of the spectrum with RF artifacts. On the other hand, in this aspect, the combination of phase contrast acquisition, which needs to be acquired during the delivery of the stimulus in order to capture the muscle movement, and 31P spectroscopy, which needs to happen in the quiescent phase, is particularly well-suited. The sequence delivered good image and spectrum quality, and the next step will be its integration into a clinical investigation to correlate the dynamic and the metabolic information.
Conclusion
An interleaved 1H phase contrast imaging and 31P spectroscopy sequence was successfully implemented and tested during electrical muscle stimulation delivering good image and spectral quality. These results are a step towards a comprehensive functional muscle investigation protocol.Acknowledgements
We would like to thank Dr A. Lopez Kolkovsky for the useful discussions on the sequence implementation.
This work was supported by the Swiss National Science Foundation (Nr. 172876) and the Lorenzo Piaggio Foundation.
References
1. Deligianni X, Pansini M, Garcia M, et al. Synchronous MRI of muscle motion induced by electrical stimulation. Magn Reson Med. 2017;77(2):664-672.
2. Matheson GO, McKenzie DC, Gheorghiu D, Ellinger DC, Quinney HA, Allen PS. 31P NMR of electrically stimulated rectus femoris muscle: an in vivo graded exercise model. Magn Reson Med. 1992;26(1):60-70.
3. Lopez Kolkovsky AL, Marty B, Giacomini E, Carlier PG. Feasibility study of interleaved multi-nuclear acquisitions on a 3 T clinical NMR scanner without hardware modifications. In: Proceedings 25th Scientific Meeting, International Society for Magnetic Resonance in Medicine. ; 2016.
4. Dumoulin CL, Souza SP, Darrow RD, Pelc NJ, Adams WJ, Ash SA. Simultaneous acquisition of phase-contrast angiograms and stationary-tissue images with Hadamard encoding of flow-induced phase shifts. J Magn Reson Imaging. 1991;1(4):399-404.
5. Santini, Francesco, Fischer, Dirk, Bieri, Oliver, Deligianni, Xeni. Dynamic 31P spectroscopy during superimposed electrical muscle stimulation and volitional contraction for enhanced metabolic response in the skeletal muscle. In: Proceedings 25th Scientific Meeting, International Society for Magnetic Resonance in Medicine. Honolulu, HI.
Figures