5130
Altered Microcirculation and Oxygenation of Skeletal muscle in Type 2 Diabetes Mellitus (T2DM) Rhesus Monkeys with Non-contrast MRI Perfusion and Oximetry Techniques1West China Hospital, Sichuan University, Sichuan 610041, China;, Chengdu, China, 2Sichuan Primed Bio-Tech Group Co., Ltd., Chengdu, China, Chengdu, China, 3Mallinckrodt Institute of Radiology, Washington University School of Medicine, St. Louis, Missouri, USA, St. Louis, MO, United States
Synopsis
In this study, the altered microcirculation and oxygenation of skeletal muscle in T2DM rhesus monkeys were evaluated by non-contrast skeletal muscle MR perfusion and oximetry techniques. We found that the perfusion of skeletal muscle decreased, especially in fast-twitch fiber muscles, and with an air-cuff caused muscle hyperemia, the ability to reperfusion in slow-twitch muscle is higher than in fast-twitch muscle. The oxygen extraction fraction of skeletal muscle significantly increased in all skeletal muscle angiosomes. These results suggest the diverse adaptation of slow- and fast-twitch skeletal muscles to T2DM.
introduction
Microcirculation of the diabetic foot has proven a central role in the development of diabetic foot syndrome and their subsequent failure to heal. And the abnormalities in vascular reactivity in the microcirculation are present even at an early stage in T2DM1. In this study, we use non-contrast skeletal muscle MR perfusion and oximetry techniques to quantify different adaptation of microcirculation and oxygenation in skeletal muscle to type 2 diabetes mellitus (T2DM) in diabetes rhesus monkey.Methods
Ten previously screened spontaneous T2DM rhesus monkeys (FPG, 6.86±1.76 mmol/L; HbA1c, 5.99±2.11%) were matched with eight age-matched, healthy monkeys with well-controlled glucose level (FPG, 4.30±0.28mmol/L; HbA1c, 4.21±0.13%). The skeletal muscle blood flow (SMBF) and oxygen extraction fraction (SMOEF) measurements were performed with an air-cuff protocol (4 min at rest, 4 min inflation, and 4 min deflation periods) on a clinical 3.0T Siemens Trio scanner. The cuff was placed on the mid-thigh above the right knee. One section was centered at the largest cross-section of the calf for SMBF measurements, but 3 sections in the calf muscle were measured for the mean SMOEF measurements. The SMBF (ml/min/100g) was measured using an arterial spin labeling method only during the recovery period for hyperemic flow with a temporal resolution of 20 sec and a division of the resting SMBF was made to achieve the relatively changeable SMBF standardized all of the SMBF data. The SMOEF maps were measured using a susceptibility-based MRI technique with a temporal resolution of 4 min. Three region of interests were placed on the triceps surae muscle, tibialis anterior muscle and tibialis posterior muscle of the images for quantitative SMBF and SMOEF measurements. Two T2DM monkeys and two healthy monkeys were sacrificed after MRI scanning for the ATPase staining and HE staining.Results
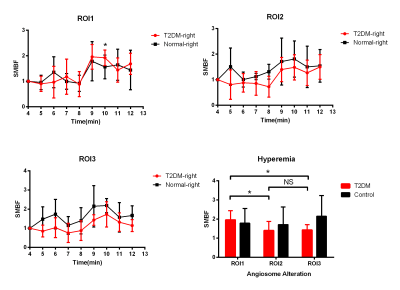
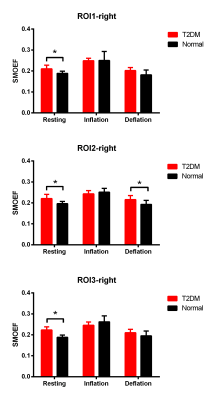
With the air-cuff applied to the right thigh, the right calf muscles of all subjects showed a progressive signal alternation after air-cuff inflation (Figure 1). The SMBF of the right leg in T2DM in tibialis anterior muscle and tibialis posterior muscles both were lower than healthy monkeys at each time-points. But for triceps surae muscle, there was not obvious blood flow decreased during inflation stage, except the significant difference observed at the 10th min. For the 9th min, which would be a hyperemia in each muscle, the hyperemia SMBF in triceps surae muscle was significantly higher than tibialis anterior muscle and tibialis posterior muscle (triceps surae muscle vs tibialis anterior muscle, 1.96±0.48 vs 1.39±0.48; triceps surae muscle vs tibialis posterior muscle 1.96±0.48 vs 1.43± 0.27, respectively; both p<0.05) (Figure 1) in the T2DM group. The SMBF during hyperemia and deflation in T2DM were significantly higher than SMBF when air-cuff inflation in all angiosomes. For SMOEF, in resting stage, the SMOEF in T2DM in all angiosomes were significantly higher than normal monkey. With the air-cuff inflation, the SMOEF in all angiosomes increased but no significant difference to be observed. After relaxing the cuff, the SMOEF returned to higher than normal, but no significant difference was observed (Figure 2). The ATPase staining of the skeletal muscle sections confirmed that triceps surae muscle is predominantly the type 1 (slow-) fiber while the others are predominantly the type 2 (fast-) fiber.Discussion
Skeletal muscle consists of slow-twitch (type1) and fast-twitch (type 2) fibers. The fiber type composition has a major influence on vascularization, capillary exchange capacity, and vascular structure and different response to the disease2,3. Undergoing the T2DM, the SMBF decrease, especially in fast-twitch fiber muscles. And with an air-cuff caused muscle hyperemia, the ability to reperfusion in triceps surae muscle is higher than in tibialis anterior muscle and tibialis posterior muscle. Meanwhile, the SMOEF significantly increase in all angiosomes, suggesting that muscle try to maintain the oxygen consumption rate when OEF is up and SMBF is down by using Fick's law.conclusion
Non-contrast MRI perfusion and oximetry techniques can quantitatively demonstrate the different impaired degrees of peripheral perfusion and oxygenation in the triceps surae muscle, tibialis anterior muscle and tibialis posterior muscle, suggesting the diverse adaptation of slow- and fast-twitch skeletal muscles to T2DM.Acknowledgements
No acknowledgement found.References
1. Greennan RL, Panasynk S, Wang X et al. early changes in the skin microcirculation and muscle metabolism of the diabetic. Lancet. 2005 Nov 12; 366(9498):1711-7.
2. Oberbach A, Bossenz Y, Lehmann S et al. altered fiber distribution and fiber-specific glycolytic and oxidative enzyme activity in skeletal muscle of patients with type 2 diabetes. Diabetes Care. 2006 Apr;29(4):895-900.
3. Jared Talbot and Lisa Maves. Skeletal muscle fiber type: using insights from muscle developmental biology to dissect targets for susceptibility and resistance to muscle disease. WIREs Dev Biol 2016, 5:518-534.
Figures