5129
Non-invasive Detection of NADH+NAD+ in Human Muscle Using 31P MR Spectroscopy at 3T1Human Magnetic Resonance Center, Institute for Applied Life Sciences, University of Massachusetts, Amherst, MA, United States, 2Department of Kinesiology, UMass, Amherst, MA, United States, 3Department of Biology, UMass, Amherst, MA, United States, 4Pioneer Valley Life Sciences Institute (PVLSI), Springfiled, MA, United States, 5Department of Medicine, University of Massachusetts Medical School (UMMS)-Baystate Regional Campus, Springfiled, MA, United States
Synopsis
NAD+ and NADH act as coenzymes in metabolic reactions. The reduction of NAD+ to NADH is linked with generation of ATP through glycolysis and oxidative phosphorylation. The consumption of NAD+ by various signaling proteins regulates protein modification, cell fate and survival. Therefore, NAD+ and NADH measurements have the potential to inform about tissue energetics and health. Recently, some investigators have suggested that NADH and NAD+ may be detected in human muscle using 31-phosphorus MRS. However, the utility and reliability of this measure is not clear. The goals of this project were to 1) determine whether the NADH+NAD peak can be resolved in human skeletal muscle at 3T, 2) compare peak resolution with and without a decoupling technique, and 3) evaluate the reliability of this measure. Interpretation of these data and their potential for studying alterations in NAD+ and NADH homeostasis in human muscle remain to be determined.
Purpose
Phosphorus magnetic resonance spectroscopy (31P MRS) is a powerful tool that enables non‐invasive quantification of changes in skeletal muscle energetic capacity1,2. NAD+ homeostasis within the cytosol, nucleus and mitochondria plays critical role in cellular energetics, epigenetics, and cell fate determinations3. Therefore, NAD+ and NADH contents may change with age and pathological states. 31P MRS-based noninvasive and quantitative measurements of NAD+ and NADH in animal and human brains have been made using high and ultra-high magnetic fields4,5 as well as in human skeletal muscle at 1.5T6. However, lower field (1.5T) does not provide enough resolution compared to 3T. Also, 3T gives better signal to noise ratio (SNR) and ability to decouple 1H from 31P. The advantage of 1H-decoupling is that it significantly narrows the line widths of NAD and α-ATP resonances, resulting in higher sensitivity and better spectral resolution compared to the 1H-coupled 31P spectrum. Here by incorporating the 1H- decoupling technique, we have investigated NAD+ and NADH peak separation and determined total NAD content within human skeletal muscle at 3T. We also investigated the within-day test-retest reproducibility.Materials and Methods
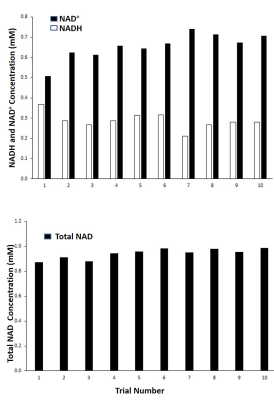
A double tuned 1H/31P 10/8 cm circular surface coil was used for the MRS acquisition. 31P MRS data were collected from vastus lateralis muscles of four healthy volunteers (mean 40.5 years) in Siemens 3T Skyra MRI scanner (Siemens Medical Solutions, Erlangen, Germany) operating on a VE11C platform. Gradient-echo scout images were used to confirm optimal positioning of the leg in the isocenter, and correct positioning of the coil over the muscle. A single-pulse-acquisition sequence with the option of 1H-decoupling (WALTZ-4: decoupling duration: 1ms) during the FID acquisition was applied to obtain 31P spectra with a hard 90° RF excitation pulse and the following parameters: 4000 Hz spectral width, 2048 number of points, 2000 ms repetition time and 64 averages (2:08 min scan time). Magnetic field homogeneity was optimized on water using the 1H coil, and confirmed on the PCr peak of the 31P signal to yield full width at half maximum (FWHM) of ~10 Hz. For intra-subject reproducibility, the same protocol was performed ten times in the vastus lateralis of one of the volunteers. Spectra were processed using jMRUI 6.0 and quantified using AMARES non-linear least squares algorithm. To evaluate reproducibility, the mean, standard deviation (SD) and coefficient of variation (CV) of 10 in vivo measurements were determined.Results and Discussion
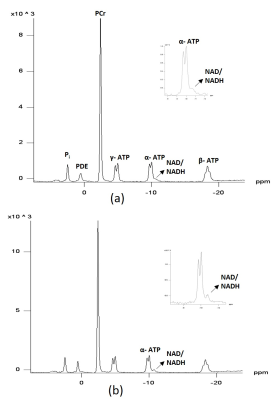
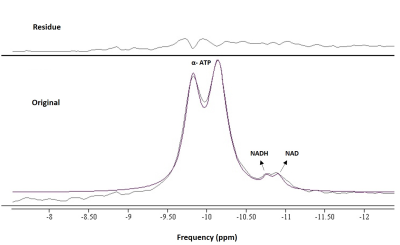
Fig.1 shows the 1H-coupled (a) and 1H-decoupled (b) in vivo 31P MR spectra of human muscles from a 63 year-old healthy subject. Inserts are the expanded spectra in the chemical shift range of −9 to −12.0 ppm (using PCr resonance at -2.5 ppm as chemical shift reference). The line fitting and residue is shown in Fig.2. Applying 1H decoupling has different effects on the appearance of 31P spectra. With 1H-decoupling, the spectral resolution in the region of the diesters has increased sensitivity. Moreover, the NAD+/NADH peak directly next to the α-ATP can be resolved. All the resonances (α-ATP, NAD+ and NADH) were satisfactorily fitted, as reflected by the small residue between the original spectrum and fitting. When using the α-ATP signal corresponding to 8.2mM (healthy muscle) as an internal standard, the intra-subject reproducibility measurement (Fig.3) of total NAD concentration was 0.96±0.07mM ( CV 7.6%) using a single peak fit and 0.94±0.04mM (CV 4.3%) using a double peak fit, which agree with earlier findings6.Conclusion
The spectra in Figures 1-3 clearly demonstrate better-resolved resonance signals and improved spectral quality of 1H-decoupled 31P spectra compared to non decoupled spectra, which could result in more reliable total NAD+ and NADH measurement. This decoupling technique needs to be validated with measurements in more subjects, and using phantoms to differentiate NAD+ from NADH. Also, prior knowledge for the decoupling technique needs to be optimized for the NAD+ and NADH quantitation. Future work to determine the utility of NAD+/NADH quantitation is needed.Acknowledgements
No acknowledgement found.References
1. Chance B, Leigh JS, Clark BJ, Maris J, Kent J, Nioka S, Smith D. Control of oxidative metabolism and oxygen delivery in human skeletal muscle: a steady-state analysis of the work/energy cost transfer function. Proceedings of the National Academy of Sciences. 1985 Dec 1;82(24):8384-8.
2. Lanza IR, Bhagra S, Nair KS, Port JD. Measurement of human skeletal muscle oxidative capacity by 31P‐MR spectroscopy: a cross‐validation with in vitro measurements. Journal of Magnetic Resonance Imaging. 2011 Nov 1;34(5):1143-50.
3. White AT, Schenk S. NAD+/NADH and skeletal muscle mitochondrial adaptations to exercise. American Journal of Physiology-Endocrinology and Metabolism. 2012 Aug 1;303(3):E308-21.
4. Zhu XH, Lu M, Lee BY, Ugurbil K, Chen W. In vivo NAD assay reveals the intracellular NAD contents and redox state in healthy human brain and their age dependences. Proceedings of the National Academy of Sciences. 2015 Mar 3;112(9):2876-81.
5. Lu M, Zhu XH, Chen W. In vivo 31P MRS assessment of intracellular NAD metabolites and NAD+/NADH redox state in human brain at 4 T. NMR in Biomedicine. 2016 Jul 1;29(7):1010-7.
6. Conley KE, Ali AS, Flores B, Jubrias SA, Shankland EG. Mitochondrial NAD (P) H In vivo: Identifying Natural Indicators of Oxidative Phosphorylation in the 31P Magnetic Resonance Spectrum. Frontiers in physiology. 2016;7.
Figures