5125
Clinically Feasible Model-based Analysis of Amide Proton Transfer MRI in Acute Ischaemic Stroke1Institute of Biomedical Engineering, University of Oxford, Oxford, United Kingdom, 2CRUK & MRC Oxford Institute for Radiation Oncology, University of Oxford, Oxford, United Kingdom, 3Wellcome Centre for Integrative Neuroimaging, FMRIB, Nuffield Department of Clinical Neurosciences, University of Oxford, Oxford, United Kingdom, 4Acute Stroke Programme, Radcliffe Department of Medicine, University of Oxford, Oxford, United Kingdom, 5School of Medicine, Faculty of Health, University of Tasmania, Hobart, Australia
Synopsis
Model-based analysis of CEST MRI is a robust quantitative method, however, the lengthy acquisition and processing times make it less clinically feasible. It has recently been proposed that partial acquisition of Z-spectra provides a faster approach, but at the cost of increased variability and large alterations in baseline Amide Proton Transfer (APT) effect. Here we present a refined approach, accounting for magnetisation transfer effects, which reduces acquisition and processing times and also decreases variability in the data. We demonstrate its ability to detect pathological reductions in the APT effect in both preclinical and clinical cohorts of acute ischaemic stroke respectively.
Purpose
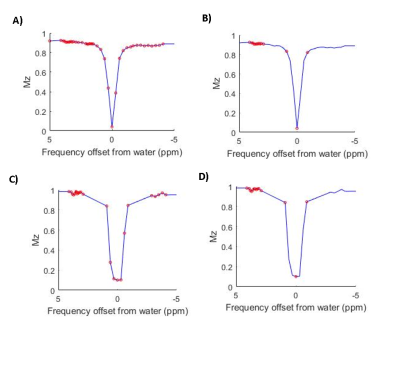
Bayesian model-based analysis of Amide Proton Transfer (APT) MRI typically requires acquisition of full Z-spectra. Therefore, whilst it quantitatively outperforms conventional APT MRI quantification methods1, it is less clinically feasible. It was demonstrated in a preclinical model of acute ischaemic stroke, that partial acquisition of Z-spectra, with sampling focused around pools of interest (Fig.1), could significantly reduce acquisition and processing times for model-based analysis2. Whilst this reduced frequency offset (RFO) approach could detect pathological alterations in the APT effect (measured using the APTR* metric3), and remained insensitive to B0 inhomogeneities, APTR* contrast between ischaemic and healthy tissue was significantly diminished. Furthermore, absolute APTR* was overestimated, with increased variability, in comparison to a fully sampled Z-spectrum. Here, we present a refined RFO approach that incorporates magnetisation transfer (MT) effects, evaluating its performance in preclinical and clinical datasets of acute ischaemic stroke.Methods
Preclinical: Data from a middle cerebral artery occlusion model of ischaemic stroke were retrospectively analysed2. In brief, focal ischaemia was induced in six Wistar rats, and MRI immediately performed using a 9.4T system (Agilent) with 72mm volume-transmit and 4-channel surface-receive array (Rapid Biomedical). APT MRI was performed with 50 Gaussian pulses (40ms duration, 184° FA, 50% duty cycle, equivalent continuous wave saturation power 0.55µT) across 51 saturation frequencies (-300-300 ppm, Fig.1A) and SE-EPI readout (TR/TE =5s/27.2ms). T1 and T2 maps were acquired using IR-EPI (TR/TE=10s/27.22ms, 9TIs:0.013-8s) and SE-EPI (TR=5s, 10 TEs:30-160ms) sequences. DWI (TR/TE=3s/27.2ms, 3 directions, b=0/1000s/mm2) and multi-phase pCASL (8 phases 0-315°, tag thickness 6.2mm, TR/TE/PLD=4s/12.4ms/550ms, bolus duration 1.4s) were acquired for identification of perfusion-diffusion mismatch. Images were acquired with 0.5x0.5x1mm3 resolution across 10 slices.
Clinical: Published data from 8 patients with ischaemic stroke (6F/2M, 73±16 years) were retrospectively analysed4. MRI was performed at 3T (Siemens Verio) within 12 hours of symptom onset according to research protocols approved by the UK National Research Ethics Service committee. APT MRI data were acquired in a single slice (3×3×5mm3 resolution), with TR/TE=5000/28 ms, with 2D-EPI readout. Clinical APT MRI preparation followed the same protocol as preclinical acquisition, except it was only acquired at 32 offsets (Fig.1). DWI, PCASL, FLAIR and MPRAGE were also acquired for infarct localisation and co-registration as previously described4.
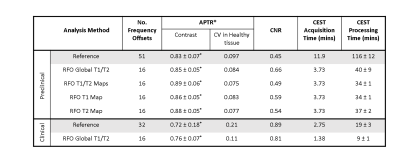
Analysis: Saturation frequency offsets were retrospectively reduced to 16 (Reduced Frequency Offset, RFO) (Fig.1). Model-based analysis was performed using FSL’s BayCEST3,5. Reference methods consisted of analysing full Z-spectra, assuming a 3-pool exchange model (water, amide, and magnetisation transfer + Nuclear Overhauser Enhancement). T1 and T2 values were initialised on a voxelwise basis for preclinical analsysis6, and on a global basis for clinical analysis (in absence of T1/T2 maps). RFO analysis assumed an alternative 3-pool model (water, amide, magnetisation transfer), with T1 and T2 initialised globally. To assess the dependence of model-based analysis results on T1 and T2, preclinical data were also analysed using voxelwise values of: (1) T1 and T2; (2) T1 only; and (3) T2 only. APTR* was calculated using fitted parameters as previously described1,3,5. Masks for ischaemic core and contralateral normal tissue were created from ADC and CBF maps in MATLAB, as previously outlined4,7,8. RFO performance was assessed using APTR* contrast (APTR*Ischaemic/APTR*Contralateral), coefficient of variation (CV) in APTR* of contralateral tissue, and APTR* contrast-to-noise ratio, CNR ((APTR*Contralateral–APTR*Ischaemic)/σContralateral). Differences were assessed using a Student’s t-test, with statistical significance defined as p<0.05.
Results
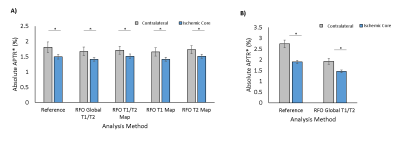
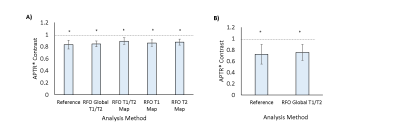
Significant reductions in APTR* were observed in the ischaemic core in both preclinical and clinical cohorts across all RFO methods (Fig.2), with no alterations in contrast between Reference and RFO methods (Fig.3). However, a significant reduction was observed between Reference and RFO estimates of absolute APTR* in clinical data and preclinical contralateral tissue in the absence of a T2 map (Table 1). RFO analysis of preclinical and clinical data reduced the CV of baseline APTR* across subjects, with no significant alterations in CNR (Table 1). All RFO methods significantly reduced processing time (Table 1).Discussion
Both preclinical and clinical results show that reducing the number of frequency saturation offsets, greatly reduces acquisition and processing time for model-based analysis of APT MRI, without diminishing pathological tissue contrast. Sampling only frequencies close to pools of interest reduces the inter-subject variability in APTR* of healthy tissue, which is promising, given a limited range of pH is expected. A remaining challenge is the observed shift in absolute APTR*, unless a T2 map is provided. However , further optimisation of the sampling schedule around the water peak would likely overcome this T2-dependence. Here we have demonstrated the clinical viability of partial Z-spectra acquisition in acute ischaemic stroke, where the emergency setting necessitates short scan times.Acknowledgements
This work was funded by the Oxford Cancer Imaging Centre, Cancer Research UK, Engineering and Physical Sciences Research Council, the National Institute for Health Research Oxford Biomedical Research Centre Programme, the National Institute for Health Research Clinical Research Network, and the Dunhill Medical Trust
References
[1] Tee et al., Comparing Different Analysis Methods for Quantifying the Amide Proton Transfer (APT) Effect in Hyperacute Stroke Patients. NMR in Biomed., 2014, 27:1019-1029
[2] Croal et al., Model-Based Analysis of Partial Z-spectra for Rapid Quantification of Amide Proton Transfer MRI. Proc. ISMRM, 2017, #3779
[3] Chappell et al., Quantitative Bayesian Model-Based Analysis of Amide Proton Transfer MRI. MRM., 2013, 70:556-567.
[4] Harston et al., Identifying the Ischaemic Penumbra using pH-weighted Magnetic Resonance Imaging. Brain. 2015; 138: 36-42.
[5] Chappell MA, Groves AR, Whitcher B, Woolrich MW. Variational Bayesian Inference for a Nonlinear Forward Model. IEEE Trans Signal Process., 2009, 223–236.
[6] Ray et al., Determination of an Optimally Sensitive and Specific Chemical Exchange Saturation Transfer MRI Quantification Metric in Relevant Biological Phantoms. NMR in Biomed., 2016, 29:1624-1633
[7] http://www.fmrib.ox.ac.uk/fsl/basil
[8] Larkin et al. Multiphase pCASL for Imaging Blood Flow in Rodent Brains, Proc. ISMRM, 2016, #5333
[9] Tee at al., Optimal Sampling Schedule for Chemical Exchange Saturation Transfer. MRM., 2013, 70(5):1251-1262
Figures