5124
Chemical exchange saturation transfer magnetic resonance imaging of functionalized poly(N, N’-methylene bisacrylamide 4-aminobutanol) gel1Radiology and Nuclear Medicine, Radboud University Medical Center, Nijmegen, Netherlands, 2Biomaterials Science and Technology, University of Twente, Enschede, Netherlands, 3Experimental Urology, Radboud University Medical Center, Nijmegen, Netherlands, 4Radiology and Radiological Science, The Johns Hopkins University School of Medicine, Baltimore, MD, United States
Synopsis
Poly (amido amine)s like poly(N, N’-methylene bisacrylamide 4-aminobutanol) (MBA-ABOL) are compounds with promising biomedical applications, which, however, require that they can be visualized without contrast application. In this study we investigated if they can be imaged in a “label free fashion” by CEST MRI making use of their exchangeable amide and hydroxyl protons. We systematically determined optimal conditions for CEST in MBA-ABOL in solution and then demonstrated that the material can be imaged both in vitro and ex vivo, implanted in a rat leg, with a strong CEST effect from the amide protons and substantial effect from the hydroxyl protons.
Introduction
Poly (amido amine)s (PAAs) are compounds with a good water solubility and biodegradability. They have been proposed for various biomedical applications including drug and gene delivery and tissue regeneration.1-3 Direct characterization of these gelly materials after injection in tissues, however is problematic and often relies on invasive procedures or doping with contrast agents with a risk of toxicity.
Chemical exchange saturation transfer (CEST) imaging,4 as an MR contrast approach relying on proton exchange between specific chemical compounds and bulk water pool, is an attractive alternative to address this problem in a “label free” fashion,5 because PAA materials include exchangeable protons in their amide (-NH) group.6
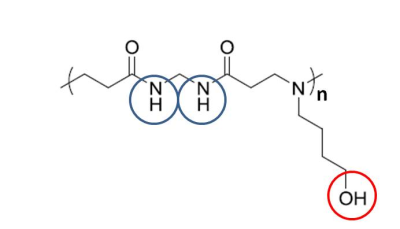
In this study a newly developed PAA material, poly(N, N’-methylene bisacrylamide 4-aminobutanol) (MBA-ABOL),7-9 which consists of exchangeable protons in amide and hydroxyl groups (Fig.1), was tested for its ability in CEST MRI. This involved the determination of optimal scan parameters to perform CEST MRI in vitro and a further ex vivo investigation of CEST imaging of this material after implanting into a rat leg.
Materials and Methods
In vitro sample
MBA-ABOL powders were dissolved in milliQ water at a low concentration of 1% by vortexing for approximately 2 minutes, and then became gelated at room temperature. Subsequently, the formed gelly solution was stored in an Eppendorf tube (1500µl).
Ex vivo sample
150µl MBA-ABOL, imbedded into a small piece of a porous collagen type I sponge,10 was implanted into the right posterior leg of a Wistar rat cadaver. To best mimic the in vivo situation, the rat was sacrificed directly before the material implantation, followed by the CEST MRI experiment.
MR Experiments
All CEST experiments were performed on an 11.7T MR system (Biospec, Bruker, Germany) using a Bruker 1H volume coil with an inner diameter of 40mm.
For the in vitro sample, a single slice CEST modified rapid acquisition with relaxation enhancement (RARE) sequence (RARE factor=12, TR/TE=4200/30ms, image resolution=0.23mm x 0.23mm x 2mm) was applied to acquire CEST images of MBA-ABOL at sweeping frequencies of ±8ppm (step=0.4ppm) at room temperature. To determine optimal CEST MRI conditions, different saturation pulse amplitudes (50, 100, 150, 200 and 250Hz) at duration of 4000ms, and different saturation pulse durations (500, 1000, 2000, 4000, 6000ms at TR=6500ms) at amplitude of 150Hz were applied. For B0 corrections,11 the same parameters were used except TR=2000ms, and a saturation pulse with amplitude/duration: 21Hz/500ms and sweeps at ±2ppm (step=0.2ppm).
For ex vivo measurement, a similarly parameterized CEST-RARE sequence was employed but with RARE factor=4, TR/TE=4025/10ms, image resolution=0.17mm x 0.17mm x 2mm and the saturation pulse with only 100Hz amplitude and 4000ms duration. For B0 corrections,11 the same parameters were used except TR=1500ms, and a saturation pulse amplitude/duration: 21Hz/500ms with sweeps at ±2ppm (step=0.2ppm).
Data analysis
The corresponding CEST images, Z-spectrum and the magnetic transfer asymmetry (MTRasym) plots were obtained with custom-written scripts in Matlab 2014b.
Results
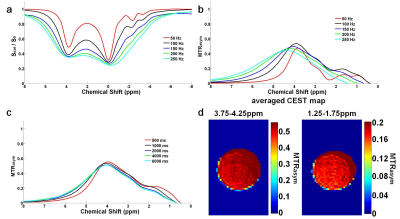
As shown in Fig.2, for the 1% MBA-ABOL solution significant CEST effects were obtained, indicated by high MTRasym (~50%-60%) at about 4ppm which we assign to arise from its amide protons. Also, a strong CEST effect with an MTRasym of ~20% was observed at about 1.5ppm especially at saturation pulses with amplitudes less than 150Hz. This effect is assigned to arise from the hydroxyl protons of the compound. Additionally, both amplitude and duration of the saturation pulse have optimal values for a maximal CEST MTRasym effect (Fig.2b,c).
Applying the optimal settings of the saturation pulse with amplitude of 100Hz and duration of 4000ms CEST MR images of the MBA-ABOL phantom was obtained with separately averaging the MTRasym from 3.75 to 4.25ppm and 1.25 to 1.75ppm (Fig.2d).
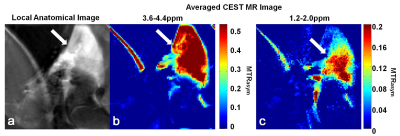
Similar to these findings we observed a notable CEST effect with a high MTRasym (~50%) at about 4 ppm and a MTRasym of around 20% at about 1.5 ppm for the MBA-ABOL implanted in a rat leg, which allowed to obtain separate CEST MR images of this material in the leg from the average MTRasym at 3.6-4.4ppm and at 1.2-2.0ppm (Fig.3).
Discussion and Conclusion
In this study we determined optimal conditions for CEST MRI of the MBA-ABOL in vitro and applied this to obtain CEST images of this material implanted in the rat leg. As the MBA-ABOL shows CEST effects both at amide and hydroxyl frequencies this may be used as a signature to separate it from CEST effects of other amide compounds. Further in vivo CEST MRI studies are ongoing to examine the real potential of this material and also to test if CEST MRI can follow the biodegradation of this material longitudinally.Acknowledgements
This project is supported by FP7-PEOPLE-2013-ITN under grant no 607868 (iTERM) and EFRO-GO (2011-020637).References
[1] Ferruti P. et al., Macromol. Rapid Commun. 23:332-55 (2002).
[2] Emilitri E. et al., J. Polym. Sci., Part A: Polym. Chem. 43:1404-16 (2005).
[3] Ferruti P. et al., Macromol. Biosci. 5:613-22 (2005).
[4] Ward K. et al. J Magn Reson. 143:79-87 (2000).
[5] Liang Y. et al. Biomaterials. 42:144-50 (2015).
[6] Goffeney N. et al. J AM. Chem. Soc. 123:8628-29 (2001).
[7] Ekkelenkamp A. et al. Acta. Biomater. 30:126-34 (2016).
[8] Lin C. et al. Bioconjugate Chem. 18:138-45 (2007).
[9] Hujaya SD. et al. Acta Biomater. 22:19-31 (2015).
[10] Sun W. et al. Tissue Eng Part C Methods. 18:731-9 (2012).
[11] Kim M. et al. Magn Reson Med. 61:1441-50 (2009).
Figures