5120
Magnetization Transfer in Lipids - Role of Exchangeable Groups and Water Binding1New York University, New York, NY, United States, 2New York University, 10003, NY, United States
Synopsis
We study the magnetization exchange mechanism in lipid systems, with relevance to imaging myelin via MT contrast. Studies of samples with different lipid compositions reveal exchange time scales, and the role of structural features in the contrast mechanism. Insights from molecular dynamics provide estimates of the contribution of the dipolar pool equilibration to the MT amplitude. The effect of lipid head groups, and the contribution of cholesterol and proteins are examined. It is hoped that these findings will help explaining the origin of White Matter MT contrast and will allow better myelin quantification by tailored saturation sequences.
Introduction
Magnetization transfer (MT) is of interest due to its ability, in principle, to detect macromolecular fractions. In White Matter, in particular, it has been suggested that myelin content can be detected via MT. Several different pulse sequences and techniques have been suggested for efficient ways of achieving specific image contrast. These include multi-frequency irradiations, and algorithms termed 'inhomogeneous magnetization transfer' [1-3]. In this contribution, we examine the role of different features of lipid membranes in producing image contrast. In particular, we study the effect of exchangeable groups, the composition in terms of cholesterol and protein content on MT effect size and provide models that detail the nature and kinetics of the MT process. A double-quantum filter experiment is used to determine the exchange time scale between the macromolecule and the liquid. The systems selected in this study are seen as good model systems for myelin in view of identifying efficient contrast for MRI in demyelinating diseases. Methods
Samples containing DMPC, DMPC+cholesterol, DMPC+cholesterol+protein, DMPC+sphingomyelin, and DMPC+cerebroside are prepared in their multilamellar phases. Phase behavior is ascertained with 31P NMR. MT experiments are performed by acquiring z-spectra with 0.5-2s irradiation at powers from 100Hz-1kHz. Double-quantum filtered experiments are acquire using the sequence describe in Ref. [4]. The sequence works by filtering the signal from a dipolar coupling pool, and then allows to measure the water signal as a function of exchange time. Molecular dynamics simulations of lipid bilayers (Fig. 1) are used to determine the behavior of the lipids and extract residual dipolar couplings, which are used to determine the transfer effect.Results and Discussion
The selection of samples allows us to test the contribution of the different components to the MT effect and to the water exchange behavior. For example, cholesterol has an exchangeable site, while DMPC does not. The role of proteins is elucidated separately as well, by examining samples with and without protein. Protein is expected to contribute significantly to MT. In addition, sphingomyelin contains -OH and -NH sites at the top of the lipid chain, but no head group with exchangeable sites. Cerebrosides contain sugar head-groups in addition
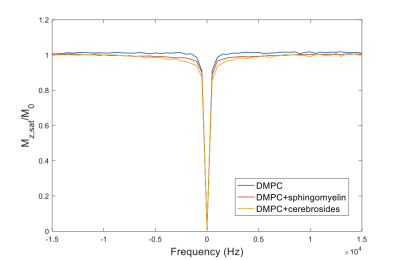
As seen in Fig. 2, there is a marked difference in MT behavior between these samples, and, in particular, the presence of exchangeable sites in the lipid chain enhances the transfer, but also the exchangeable sites in the head-group. Direct transfer to water can be neglected as a mechanism. The effect of the head-group is analyzed on the basis of the residual couplings from the dipolar pool in the lipid chain.
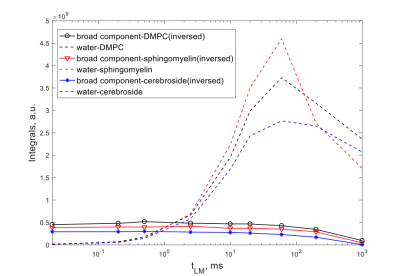
DQ filtered results produce a different kind of assessment of the exchange mechanism. As can be seen in Fig. 3, the exchange to water from different lipids occurs roughly at the same time scale for all lipids despite the nature of the head-groups, which is surprising. Faster exchange rates are seen for the cerebroside samples, but the difference is not large to explain the results simply on the basis of the presence of exchangeable sites. Inter-chain transfers can be excluded as a mechanism on the basis of lateral in-layer diffusion.
Conclusion
Systematic studies of lipid bilayers in different compositions allow us to determine the nature of the MT contrast mechanism. Insights from molecular dynamics provide estimates of the contribution of the dipolar pool equilibration to the MT amplitude. Double-quantum filtered experiments allow us to determine the origin of the contrast. The effect of lipid head groups, and the contribution of cholesterol and proteins are examined. It is seen that the MT increases with the number of exchangeable sites in the head-group. The DQ exchange experiment, however, is less understood at the moment, since it suggests that the exchange is happens on a very similar timescale regardless of the nature of the lipid. All experiments and calculation, however, indicate that the notion of isolated methylene groups is misleading, since the dipolar couplings to nearest neighbors and next-nearest neighbors are very large. It is hoped that these findings will help explaining MT and ihMT contrast mechanisms.Acknowledgements
NIH funding is acknowledged under award #5R01EB016045References
1. Manning, A. P., Chang, K. L., MacKay, A. L. & Michal, C. A. The physical mechanism of ‘inhomogeneous’ magnetization transfer MRI. Journal of Magnetic Resonance 274, 125–136 (2017).
2. Varma, G. et al. Interpretation of magnetization transfer from inhomogeneously broadened lines (ihMT) in tissues as a dipolar order effect within motion restricted molecules. Journal of Magnetic Resonance 260, 67–76 (2015).
3. Swanson, S. D. et al. Molecular, dynamic, and structural origin of inhomogeneous magnetization transfer in lipid membranes. Magn Reson Med n/a–n/a (2016). doi:10.1002/mrm.26210