5081
Electro-Magnetic Property Mapping Using Kalman Filtering with a Single Acquistion at 3.0 T and 7.0 T MRI1Electronics and Information Engineering, Korea University, Seoul, Republic of Korea, 2ICT Convergence Technology for Health and Safety, Korea University, Sejong, Republic of Korea, 3Research Institute for Advanced Industrial Technology, Korea University, Sejong, Republic of Korea, 4Korea Basic Science Institute, Cheongju, Chungbuk, Republic of Korea
Synopsis
The phase-based Electro-Magnetic (EM) MR property imaging such as Quantitative Susceptibility Mapping (QSM) and MR Electric Properties Tomography (MREPT) shows great potential clinically. The main post-processing steps in QSM and MREPT are high-pass filtering and Laplacian of MR images. They, however, cause severe artifacts and noise during conventional calculations. In this work, we propose a novel reconstruction method of EM property MRI using Kalman filter algorithm and show the utility of the proposed method by comparing the imaging results.
Introduction
Recently,Methods
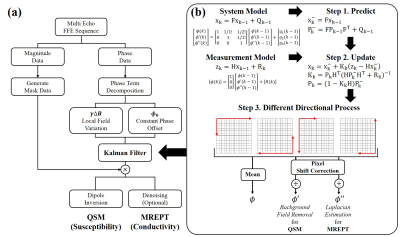
1. Kalman Filter Algorithm
To apply
To maximize the performance, the Kalman filter is applied in four different directions, which improves the SNR resulting in better estimation of differential images (Fig. 1b). Finally, we obtain the 1st and 2nd order differentials which can be used for HPF in QSM and Laplacian in MREPT, respectively.
2. Data Acquisition
In-vivo experiments were performed on a 3.0 T and
7.0 T Achieva MRI Systems (Philips, The Netherlands). Data sets were scanned
using multiple Fast Field Echo sequence and scan parameters were as follows:
TR/TE1/$$$\triangle$$$TE (ms) = 530/4.1/3.8 on 3.0 T MRI, 1345/2.6/2.3 on 7.0 T MRI, FA
= 18°, spatial resolution = 1×1×5 mm3, FOV = 256×256 mm2,
For
validation of MREPT, the Finite-Difference-Time-Domain (FDTD) simulations were
conducted using Sim4Life (ZMT AG, Zurich, CH) and Duke human model with a 12-leg
Results
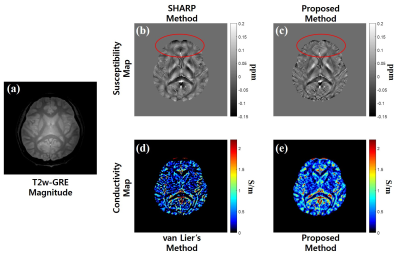
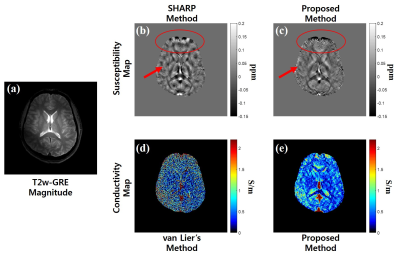
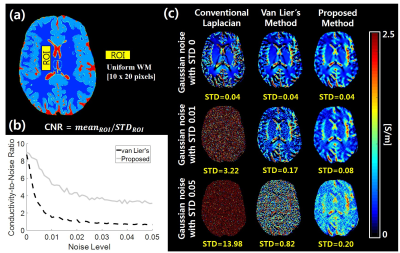
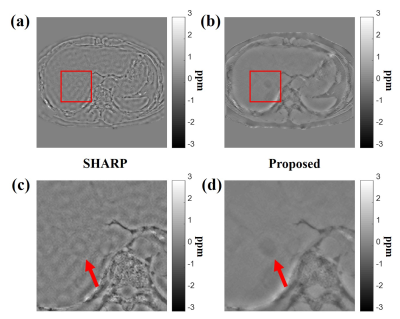
Figures 2 and 3 show the magnitude image and corresponding EM property maps computed by conventional and proposed methods at 3.0 T and 7.0 T, respectively. The mean of conductivity values are as follows: $$$\sigma_{Kalman}$$$(WM/GM/CSF) = 0.33/0.73/ 2.04 at 3.0 T, 0.31/0.77/1.31 at 7.0 T. Figure 4 shows the conductivity map reconstructed from the FDTD simulation with different noise levels whose standard deviation (STD) is 0 to 0.05. Figures 4b and c shows the conductivity-to-noise ratio and computed conductivity map when Gaussian noises of STDs of 0, 0.01, and 0.05 are added. Here the ROI was set in a uniform white matter region of 200 pixels (Fig. 4a). Figure 5 shows the abdomen QSM images obtained at 3.0 T for the whole body mask (and zoomed images) where the clear image is obtained for the proposed method compared with the conventional one.Discussion & Conclusions
For QSM, SHARP3Acknowledgements
This research was supported by the Next-generation Medical Device Development Program for Newly-Created Market of the National Research Foundation (NRF) funded by the Korean government, MSIP(NRF-2015M3D5A1065997).References
1. Reichenbach JR, et al., Quantitative susceptibility mapping: concepts and applications. Clin Neuroradiol. 2015; 25: 225-230.
2. Katscher U, et al., Recent progress and future challenges in MR electric properties tomography. Comput Math Methods Med. 2013; 546-562.
3. Schweser F, et al., Quantitative imaging of intrinsic magnetic tissue properties using MRI signal phase: An approach to in vivo brain iron metabolism. Neuroimage 2011; 54(4): 2789-807.
4. Shmueli K, et al., Magnetic susceptibility mapping of brain tissue in vivo using MRI phase data. Magn Reson Med. 2009; 62(6): 1510-1522.
5. van Lier AL, et al., B1(+) phase mapping at 7 T and its application for in vivo electrical conductivity mapping. Magn Reson Med. 2012; 67(2): 552-61.
Figures