5080
Association of thigh muscle fat infiltration with isometric strength measurements based on chemical shift encoding-based water–fat MRI1Department of Neuroradiology, Technical University of Munich, Munich, Germany, 2Department of Sport and Health Sciences, Technical University of Munich, Munich, Germany, 3Department of Radiology, Technical University of Munich, Munich, Germany
Synopsis
Chemical shift encoding-based water–fat MRI derived proton density fat fraction (PDFF) of the thigh muscles has been emerging as surrogate marker in subjects with osteoarthritis, sarcopenia, and neuromuscular disorders. However, little is known about the relationship of thigh muscle PDFF and corresponding muscle strength measurements. The present study demonstrated that PDFF measurements improve the prediction of thigh muscle strength beyond muscle cross-sectional area in healthy subjects. Thus, chemical shift encoding-based water–fat MRI can provide clinically important information and may potentially track early changes in muscles that are not severely atrophied or fattily infiltrated.
Purpose
MR-based assessment of the thigh muscles fat composition has been proposed as surrogate marker in subjects with osteoarthritis, sarcopenia, and neuromuscular disorders [1,2]. Based on chemical shift encoding-based water–fat MRI, the proton density fat fraction (PDFF) of different muscle compartments could be reliably extracted [3]. However, little is known about the relationship between thigh muscle PDFF and muscle strength measurements [4]. Specifically, it is not known whether PDFF of extensor and flexor thigh muscle groups improves the prediction of thigh muscle extension and flexion strength beyond muscle cross-sectional area (CSA) in healthy subjects. Therefore, we investigated the association of thigh muscle fat with isometric strength measurements in healthy volunteers using chemical shift encoding-based water–fat MRI.Methods
Subjects: Twenty healthy subjects (ten males, ten females; age: 29±6 years, BMI: 26.9±2.4 kg/m²) were recruited for this study.
MR Imaging: The thigh musculature of the subjects was scanned on a 3 T whole-body scanner (Ingenia, Philips Healthcare, Best, Netherlands) using the built-in-the-table posterior coil elements (12-channel array). An axially-prescribed six-echo 3D spoiled gradient echo sequence was used for chemical shift encoding-based water–fat separation. The sequence acquired the six echoes in a single TR using non-flyback (bipolar) read-out gradients and the following imaging parameters: TR/TEmin/ΔTE = 6.4/1.1/0.8 ms, FOV = 220x401x252 mm3, acquisition matrix = 68x150, voxel size = 3.2x2.0x4.0 mm³, frequency encoding direction = L/R, no SENSE, scan time = 1min and 25 s per stack. A flip angle of 3° was used to minimize T1-bias effects [5]. Two stacks were acquired to cover the entire thigh.
Imaging-Based Fat Quantification: The gradient echo imaging data were processed on-line using the fat quantification routine of the vendor. The routine performs first a phase error correction and then a complex-based water–fat decomposition using a pre-calibrated seven-peak fat spectrum and a single T2* to model the signal variation with echo time. PDFF maps were then computed as the ratio of the fat signal over the sum of fat and water signals.
Manual Segmentation: Manual segmentation of the quadriceps and ischiocrural muscles was performed bilaterally on the PDFF maps by using the free open-source software MITK. Segmentations were performed in the ten most central slices depicting the thigh muscles (Fig. 1). Mean cross-sectional area (CSA) and PDFF of the quadriceps and ischiocrural muscles were extracted bilaterally. Furthermore, the entire thigh contour was segmented in the same slices and mean thigh CSA was calculated. Relative quadriceps and ischiocrural muscle CSA was determined by dividing the respective muscle CSA by entire thigh CSA.
Isometric Strength Measurements: Muscle flexion and extension maximum isometric torque [Nm] at the thigh was measured with a rotational dynamometer (Isomed 2000). The absolute flexion and extension muscle strength measurements were adjusted for BMI to obtain relative values [Nm³/kg].
Results
Mean muscle PDFF was lower in males than females in the right and left quadriceps muscle (1.7±1.3% vs. 2.7±1.4% (p=0.105); 2.7±1.3% vs. 3.6±1.3% (p=0.162)) and ischiocrural muscle (2.6±1.9% vs. 4.9±2.4% (p=0.025); 3.2±1.6% vs. 4.6±2.0% (p=0.091)). Males showed greater relative mean muscle CSA than females in the right and left quadriceps muscle (25.8±4.2% vs. 21.8±3.7% (p=0.035); 26.5±4.0% vs. 21.3±4.1% (p=0.010)) and ischiocrural muscle (7.5±3.0% vs. 6.6±1.5% (p=0.418); 8.3±2.4% vs. 6.0±2.4% (p=0.045)). Relative muscle extension strength was greater in males compared to females in the right (8.8±1.3 Nm³/kg vs. 6.4±0.9 Nm³/kg (p<0.001)) and left thigh (8.0±1.2 Nm³/kg vs. 5.9±1.0 Nm³/kg (p=0.001)). Similarly, males had greater relative muscle flexion strength than females bilaterally (right: 4.3±0.6 Nm³/kg vs. 2.7±0.7 Nm³/kg (p<0.001); left: 4.0±1.2 Nm³/kg vs. 5.9±1.0 Nm³/kg (p<0.001)). Pearson correlation coefficients r for muscle PDFF and CSA values versus muscle strength measurements are shown in Fig. 2 and 3. In contrast to relative CSA, muscle PDFF correlated significantly with relative muscle strength. PDFF, but not relative CSA, was a statistically significant (p<0.05) predictor of relative muscle strength in multi-variate regression models except for left relative extensor muscle strength. In this case, quadriceps PDFF (p=0.005) and relative quadriceps CSA (p=0.015) contributed significantly to the statistical model with R²adj = 0.548.Discussion & Conclusion
In contrast to relative CSA, PDFF of the quadriceps and ischiocrural muscles was significantly associated bilaterally with extension and flexion physical strength measurements, respectively. Thus, water–fat MRI could detect changes of muscular fat content that correlate with muscle strength for both extensor and flexor thigh muscle groups. This may help to initiate early, individualized therapy protocols in order to maintain or improve muscle function.Acknowledgements
The present work was supported by the European Research Council (grant agreement No 637164 – iBack) and Philips Healthcare.References
[1] Kumar et al. Quadriceps intramuscular fat fraction rather than muscle size is associated with knee osteoarthritis. Osteoarthritis Cartilage 2014; 22:226.
[2] Dahlqvist et al. Severe paraspinal muscle involvement in facioscapulohumeral muscular dystrophy. Neurology 2014; 83:1178.
[3] Hu et al. Quantitative proton MR techniques for measuring fat. NMR Biomed 2013; 26:1609.
[4] Baum et al. Association of quadriceps muscle fat with isometric strength measurements in healthy males using chemical shift encoding-based water-fat magnetic resonance imaging. J Comput Assist Tomogr. 2016; 40:447.
[5] Karampinos et al. T1-corrected fat quantification using chemical shift-based water/fat separation: application to skeletal muscle. Magn Reson Med 2011; 66:1312.
Figures
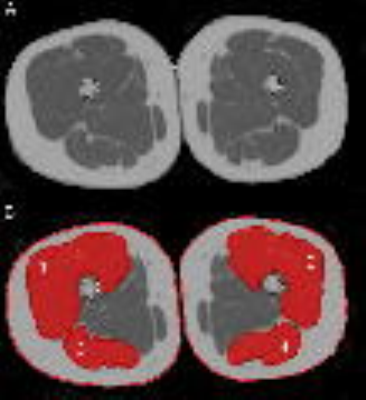
Fig. 1:
Representative PDFF map (A) and superimposed manually segmented muscle compartments (B) with 1: right quadriceps muscle, 2: left quadriceps muscle, 3: right ischiocrural muscle, and 4: left ischiocrural muscle. The red lines around the entire thigh contour were used to compute entire thigh CSA.

Fig. 2:
Plots of muscle PDFF and relative CSA versus muscle strength measurements.
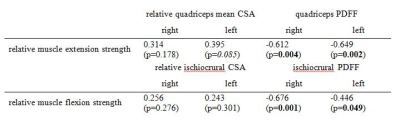
Fig. 3:
Pearson correlation coefficients r for muscle PDFF and relative CSA versus muscle strength measurements. Bold denotes p<0.05 (statistical significance), Italic denotes p<0.1 (statistical trend).