5075
Skeletal muscle acetylcarnitine in fasting and postprandial state: 1H MRS 7T pilot study.1High-Field MR Center, Department of Biomedical Imaging and Image-Guided Therapy, Medical University of Vienna, Vienna, Austria, 2Christian Doppler Laboratory for Clinical Molecular MR Imaging, Vienna, Austria, 3Division of Endocrinology and Metabolism, Department of Internal Medicine III, Medical University of Vienna, Vienna, Austria, 4Center for Biomedical Imaging, Department of Radiology, New York University School of Medicine, New York City, NY, United States
Synopsis
Acetylcarnitine plays an important role in fat metabolism. A long TE proton magnetic resonance spectroscopy was applied for detection of skeletal muscle acetylcarnitine during the day in fasting and postprandial conditions at whole body 7T MRsystem in the vastus lateralis muscle. Our observation points towards big variations of acetylcarnitine in postprandial state and no significant changes in acetylcarnitine concentrations during the fasting. Moreover, excellent repeatability of the acetylcarnitine 1H MRS based measurement was estimated during three different days in three weeks. Our data emphasize the need for strict standardization of dietary conditions and time point for the measurement of acetylcarnitine.
Introduction
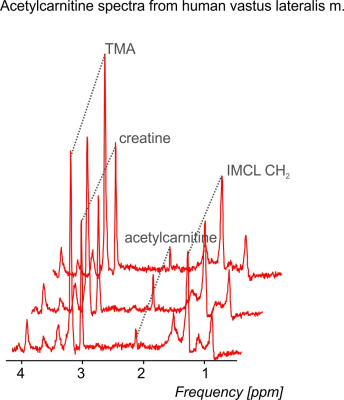
Acetylcarnitine plays an important role in fat metabolism and could be observed at 2.13ppm in long echo time 1H MR spectra in skeletal muscle[1][2]. Since carnitine concentrations in plasma are known to vary during the day[3] and following the exercise we aimed to assess the acetylcarnitine variability in the human vastus lateralis muscle using proton magnetic resonance spectroscopy at 7T and (i) to observe the behavior of acetylcarnitine in postprandial state without strenuous exercise, (ii) to observe the behavior of acetylcarnitine in fasting condition and (iii) to determine the repeatability at different days.Materials and Methods
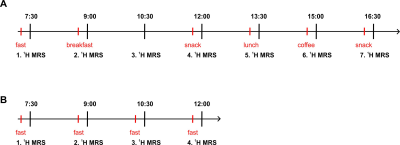
All measurements were performed on a 7T whole-body Magnetom MR system (Siemens Healthineers, Erlangen, Germany) with 28-channel knee coil (QED, Mayfield Village, OH, USA). Five healthy volunteers (Age 27±3years, BMI 22.6±2.2kg/m2, SEX 3F/2M, normal trained) were examined during the day after mixed meal ingestion, four healthy volunteers (Age 29±3years, BMI 22.4±1.6kg/m2, SEX 3F/1M, normal trained) were examined during the day in fasting state and three volunteers (Age 31±1years, BMI 21.5±2.7kg/m2, SEX 2F/1M, normal trained) participated in the assessment of the repeatability. For examination of acetylcarnitine concentrations in postprandial conditions, measurements were performed every one and half hour from 7:30 a.m. till 16:30 p.m. All the participants consumed an equal amount of the same mixed meal during the day always approximately 15 minutes before 1H MRS scan. All of them underwent the first 1H MRS scan after a twelve hour overnight fast early in the morning (7:30 a.m.), and without any exercise in the morning and day before. A schematic illustration of the experimental design, including the time points of 1H MRS, is shown in Figure 1 A. For the observation of acetylcarnitine in fasting state, measurements were performed from 7:30 a.m. till 12:00 p.m., in total four 1H MRS scans. (Figure 1 B). For the assessment of repeatability of acetylcarnitine measurements, all measurements were conducted at 7:00 a.m. in fasting state during three different days in three weeks (1 measurement per week). Volunteers did not perform any exercise during the measurement day and one day before and were in fasting state from 8 p.m. of previous day. To assess the repeatability of acetylcarnitine, the mean coefficient of variation (CV) was calculated from those three measurements. All volunteers were positioned supine in the magnet and the measurement was performed on the left vastus lateralis muscle with MRS VOI (40x35x15mm3) carefully placed into the muscle. Localized shimming was performed manually, on the adjustment volume matching the VOI size. Data were acquired using STEAM localization sequence with following parameters: TR/TE=2000/350ms; spectral bandwidth=3kHz; number of averages=128; delta frequency=-2.5ppm relative to water resonance; 4 preparation scans. For absolute quantification determination, water signal was measured separately (TR/TE=2000/20 ms; NA=1; delta frequency=0ppm) from same VOI. All spectra were fitted using the AMARES fitting algorithm in the jMRUI v5.2 software[4]. Lipids surrounding the acetylcarnitine peak were fitted with a constrained frequency of 2.0 - 2.1ppm and 2.17-2.30ppm. Using the water peak as an internal reference, the concentration of acetylcarnitine was calculated for millimolar concentration in wet weight (mmol/kg ww). Differences in the values of the acetylcarnitine concentration during postprandial and fasting state were tested for significance by repeated-measures ANOVA and LSDcorrected post-hoc test in SPSS (version 24.0; IBM SPSS, Chicago, Illinois, USA). All values are provided as mean ± SD.Results
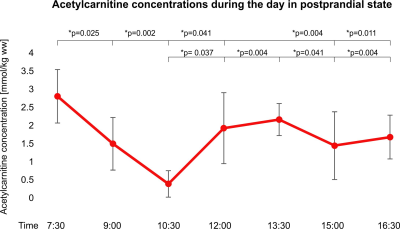
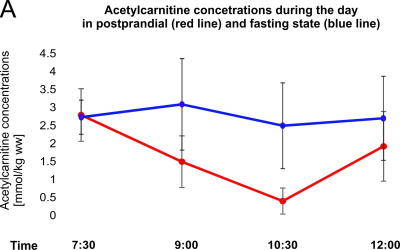
Acetylcarnitine concentrations significantly changed in the postprandial state, being highest in the morning in fasting state (2.80 ± 0.74mmol/kg ww) and lowest about two hours after breakfast (0.38 ± 0.37 mmol/kg ww)(Figure 2). Later on (12:00 o’clock) acetylcarnitine concentrations rose again and remained higher than at 10:30 a.m. throughout the whole afternoon. Acetylcarnitine concentrations did not significantly change in fasting state (mean 2.79 ± 0.99 mmol/kg ww) (Figure 3). The mean individual coefficient of variation estimated in three volunteers from three different days in three different weeks was 29.03% (Figure 4).Discussion and Conclusions
Our results demonstrate significant changes of acetylcarnitine concentrations in human vastus lateralis muscle during the day with normal food ingestion but no changes in concentrations during in fasting state. Thus we can suggest the most suitable conditions for acetylcarnitine measurement the morning hours in fasting state where the levels of acetylcarnitine are most stable. Our data emphasize the need for strict standardization of dietary conditions for the measurement of the acetylcarnitine.Acknowledgements
Colleagues at the MR Centre of Excellence, Anniversary Fund of Austrian National Bank (OeNB) ( #15363 to MKrs), Christian Doppler Society (to ST)References
[1] L. Lindeboom, C. I. Nabuurs, J. Hoeks, B. Brouwers, E. Phielix, M. E. Kooi, M. K. C. Hesselink, J. E. Wildberger, R. D. Stevens, T. Koves, D. M. Muoio, P. Schrauwen, and V. B. Schrauwen-Hinderling, “Long-echo time MR spectroscopy for skeletal muscle acetylcarnitine detection.,” J. Clin. Invest., vol. 124, no. 11, pp. 4915–25, 2014.
[2] R. Klepochová, L. Valkovič, M. Gajdošík, T. Hochwartner, H. Tschan, M. Krebs, S. Trattnig, and M. Krššák, “Detection and Alterations of Acetylcarnitine in Human Skeletal,” Invest. Radiol., vol. 0, no. 0, pp. 1–7, 2017.
[3] E. De Palo, R. Gatti, C. Crivellaro, C. De Palo, and C. Scandellari, “Plasma carnitine and acetyl-carnitine levels at different times of the day.,” Clin. Physiol. Biochem., vol. 5, no. 2, pp. 95–102, 1987. [4] L. Vanhamme, van den Boogaart A, and Van Huffel S, “Improved method for accurate and efficient quantification of MRS data with use of prior knowledge,” J. Magn. Reson., vol. 129, no. 1, pp. 35–43, Nov. 1997.
Figures