5072
SVD Compression for Quantification of 31P Relaxation Time and Creatine Kinase Reaction Rate by 31P Magnetic Resonance Fingerprinting1Department of Biomedical Engineering, Case Western Reserve University, Cleveland, OH, United States, 2Department of Radiology, Case Western Reserve University, Cleveland, OH, United States, 3Department of Physiology and Biophysics, Case Western Reserve University, Cleveland, OH, United States
Synopsis
Magnetic resonance fingerprinting (MRF) provides the opportunity for efficient quantification of ATP synthesis using 31P magnetization transfer (MT) spectroscopy. However, the multi-compartment, multi-parametric nature of 31P MT experiments renders dictionary-matching computationally infeasible. In this study, singular value decomposition was employed for parameter estimation in a 31P MRF study that quantified creatine kinase activity. Such approach allowed dictionary compression by 16 fold and accelerated parameter matching by up to 80% without compromising matching accuracy. In vivo experiments on rat hindlimb (N=21) showed a 2.7-fold increase in measurement efficiency comparing to the conventional MT method using saturation transfer.
Background
Creatine kinase (CK) plays an important role in energy metabolism in brain and muscle. 31P magnetization transfer spectroscopy (MT-MRS) allows in vivo quantification of ATP synthesis rate via CK (kfCK). However, clinical application of 31P MT-MRS is hindered by long data acquisition time. Recently, we developed a magnetic resonance fingerprinting (MRF) based method, the CK-MRF method, for improved measurement efficiency of kfCK (1). To reduce the computation time and memory requirements, only four parameters, namely kfCK, T1 of phosphocreatine (T1PCr), chemical shift of PCr (ωPCr), and PCr-to-ATP ratio (MRPCr) were matched to a dictionary, with the other parameters using fixed values from pilot measurements. However, simulation study showed that discrepancy in T1 of γATP (T1γATP) can cause significant error in kfCK estimation. Hence, variations in T1γATP under pathophysiological conditions need to be carefully evaluated. In the current study, we employed singular value decomposition (SVD) for dictionary compression and fast parameter matching (2). The efficiency and reproducibility of using SVD for parameter estimation in CK-MRF studies, including the estimation of T1γATP, were evaluated.Methods
CK-MRF data
CK-MRF data, acquired previously from the hindlimb of Sprague-Dawley rats (N=21) undergoing an ischemia/reperfusion (IR) protocol, were reanalyzed. Details of the pulse sequence and experimental protocol were described previously (1). The fingerprint comprised of 320 acquisitions of PCr and γATP signals in 20 s. For each rat, 96 and 48 single-average CK-MRF fingerprints were acquired before and after IR, respectively. For validation, 12 and 8 single-average MT-MRS datasets were also acquired before and after IR, respectively. Acquisition time for each MT-MRS dataset was 150 s.
Dictionary generation
Fingerprints were simulated by solving the modified Bloch-McConnell equation. Five parameters (kfCK, T1PCr, T1γATP, ωPCr, and MRPCr) were varied over their physiological ranges to generate the dictionary. The ranges for kfCK, T1PCr, ωPCr, and MRPCr were the same as those used in previous study. T1γATP varied from 0.5 to 2.0 s with 0.1-s increment. A total of 8,796,480 entries were simulated to generate the dictionary.
Parameter matching
Parameter matching was performed using both the uncompressed and the SVD-compressed dictionary. The uncompressed matching followed the standard MRF approach. For the compressed matching, SVD was first applied to the dictionary. Subsequently, both the CK-MRF data and the normalized dictionary were compressed using the 20 most significant left singular vectors, resulting in a 16-fold reduction in size. Parameter matching was then performed in the compressed domains.
Results
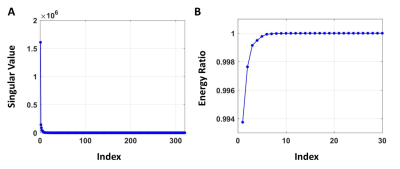
As shown in Fig. 1, singular values of the dictionary decreased rapidly (Fig. 1A) and the energy ratio associated with the first 30 singular values quickly reached 1 (Fig. 1B), suggesting the low-rank nature of the dictionary. Using the first 20 singular values, the compressed dictionary used only 6.25% of the memory required by the full-size dictionary. Compressed matching also reduced computation time up to 80%.
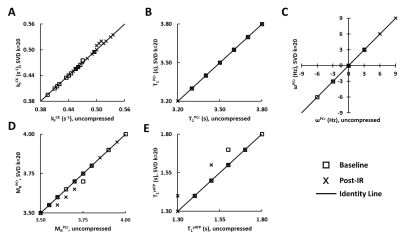
Compressed matching was first evaluated using high-SNR fingerprints obtained by averaging the data acquired before and after IR, corresponding to 96 and 48 averages, respectively. Results of compressed matching showed strong agreement with those of uncompressed matching (Fig. 2). For the estimation of kfCK, only four data points showed a mismatch that was within two dictionary steps (<0.01 s-1). Matching of T1PCr and ωPCr showed no difference between the two methods, while matching errors for MRPCr and T1γATP was within one dictionary step. Matched T1γATP was 1.56±0.12 s at baseline, which was in agreement with literature report (3) but longer than that used in previous study (1). Comparing to conventional MT-MRS, both methods showed an increase in post-IR kfCK (CK-MRF: 0.44±0.03 versus 0.50±0.03 s-1; MT-MRS: 0.39±0.03 versus 0.44±0.04 s-1; p<0.05).
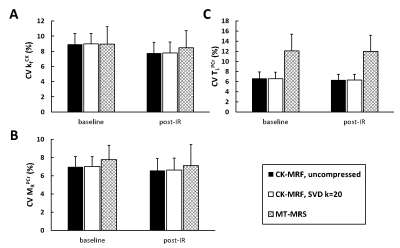
Parameter matching using single-average fingerprints also showed strong agreement between compressed and uncompressed matching. Comparing to MT-MRS, coefficients of variation (CV) was similar for kfCK and MRPCr measurements, but was significantly smaller for T1PCr measurement (Fig. 3). Considering the difference in acquisition time (20 versus 150 s), the current CK-MRF method showed at least 2.7-fold increase in measurement efficiency ($$$1/(CV\times\sqrt{ACQtime})$$$) comparing to conventional MT-MRS method.
Conclusion
In the current study, we demonstrated the utility of SVD for dictionary compression and parameter matching in CK-MRF studies. The significant reduction in memory requirements and computation time allowed the matching of additional parameters such that fewer assumptions are necessary in dictionary construction and parameter estimation. Such improvement provides the opportunities for assessing metabolic alterations in pathological conditions by CK-MRF.Acknowledgements
This work made use of High Performance Computing Resource in the Core Facility for Advanced Research Computing at Case Western Reserve University. This work was supported in part by the National Institute of Health (R01-EB023704 and R21-HL126215).
References
1. CY Wang et al. 31P magnetic resonance fingerprinting for rapid quantification of creatine kinase reaction rate in vivo. NMR Biomed 2017. doi: 10.1002/nbm.3786
2. DF McGivney et al. SVD compression for magnetic resonance fingerprinting in the time domain. IEEE Med Img 2014. doi: 10.1109/TMI.2014.2337321
3. J Ren et al. Exchange kinetics by inversion transfer: integrated analysis of the phosphorus metabolite kinetic exchanges in resting human skeletal muscle at 7 T. MRM 2014. doi: 10.1002/mrm.25256