5060
Effectiveness of Diffusion-Tensor Imaging adapted to chemical-shift-encoded water-fat MRI in dystrophic skeletal muscle1Diagnostic and Interventional Radiology and Nuclear Medicine, University Medical Center Hamburg-Eppendorf, Hamburg, Germany, 2Childrens’ Medical Center Dallas, Dallas, TX, United States, 3Medical Biometry and Epidemiology, University Medical Center Hamburg-Eppendorf, Hamburg, Germany, 4Neurology, University Medical Center Hamburg-Eppendorf, Hamburg, Germany, 5MR Clinical Science, Philips Medical Systems, Hamburg, Germany, 6University Medical Center Hamburg-Eppendorf, Hamburg, Germany
Synopsis
Diffusion tensor imaging of the skeletal muscle has been successfully applied in various conditions such as inflammation, age, and trauma. However it remains challenging in dystrophic skeletal muscle as reliable acquisition of tensor metrics is hampered due to fatty infiltration. This study aimed to assess the effectiveness of a simple clinical approach on region-of-interest localization choosing either a custom, whole muscle ROI or a selective ROI excluding areas of fatty replacement. The effect of ROI localization on tensor metrics was calculated based on mixed effect models.
INTRODUCTION
Diffusion tensor
imaging (DTI) has been promoted as a sensitive and non-invasive tool for
detection and assessment of these subtle processes caused by trauma,
inflammation or dystrophic processes, reflected by alterations in tissue
diffusivity1-4. However the application of DTI for dystrophic skeletal
muscle is challenging, as high signal-to-noise is needed to obtain accurate
estimates of DTI metrics. Previous studies demonstrated that the reliability of
estimated DTI parameters is affected by the percentage of fat in muscle, higher
percentages resulting in overestimation of FA and underestimation of ADC5-7.
The aim of this
study was to assess the influence of two different region of interest (ROI)
selections on muscle fat and DTI metrics in the thigh muscles of patients with
muscular fatty replacement due to dystrophic and myopathic conditions using a)
a whole-muscle custom ROI including, and b) a selective ROI excluding, areas of
fatty replacement on geometrically co-registered axial T1-weighted images and
FA maps.
METHODS
This IRB approved prospective study was performed between May 2014 and March 2017. Written informed consent was obtained from each case and control prior to MRI. The study cohort consisted of 15 cases (45.7 ± 19.3 y; m:f 8:6) with diagnosed muscular dystrophy and 13 healthy controls.
MRI-scans of the thigh were performed on a 3.0-Tesla (T) MRI system (Ingenia, Software Release 5.1.7, Philips, Best, Netherlands) using
(i) axial T2-weighted turbo spin echo sequence with modified Dixon fat-water separation
(ii) axial 3D GRE modified Dixon sequence
(iii) DTI using an axial fat-suppressed multislice spin-echo single-shot echo planar imaging sequence
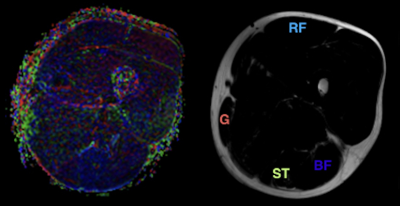
An offline workstation and software (FiberTrak; release V2.1.3 Philips Healthcare, Cleveland, Ohio) was used to derive quantitative DTI-metrics. Geometrically co-registered axial DTI and GRE images were used to identify muscles and visualize fatty replacement (Fig. 1). Three ROIs were drawn for the rectus femoris (RF), semitendinosous (ST), biceps femoris (BF), and gracilis (G) muscle by two operators by consensus using
(i) custom ROI covering the whole muscle
(ii) selective ROI excluding areas of fatty infiltration.
The percentage muscle fat fraction (MFF) was calculated using the signal intensity (SI) of three ROIs chosen for muscle and ROI localization on a custom imaging software (OsiriX; version 6.5; Pixmeo).
Quantitative MFF were calculated as $$(SIFAT/ (SIFAT+SIWATER)) x 100$$ and reported as mean value of all pixels within a ROI. The effect of ROI localization on tensor metrics was calculated based on mixed effect models.
RESULTS
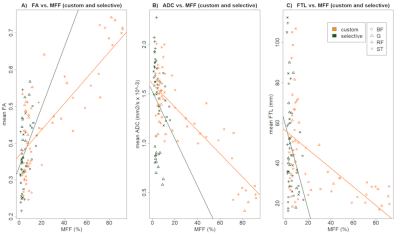
Using mixed effects models, the ADC and FA differed significantly between cases and controls on custom analysis, but not the FTL. All custom and selective analyses were highly correlated.
The correlation between custom DTI metrics and MFF in cases was significant for ADC (r = -0.27; 95% confidence interval -0.49 to -0.01) and FTL (r = -0.37; 95% CI -0.57 to -0.13), but not FA (r = 0.18; 95% CI -0.08 to 0.41). A significant correlation of ADC and FTL to MFF was also observed in controls.
No significant association between DTI metrics and MFF was observed using selective ROI in cases (ADC r= 0.10; 95% CI -0.18 to 0.36; FTL r = -0.26; 95% CI -0.5 to 0.01).
Mixed model analyses showed that the observed effect of MFF on DTI metrics (FA, ADC, FTL) was not significantly influenced by the ROI-localization in ADC and FA. For FTL ROI-localization significantly influenced the negative effect of MFF.
DISCUSSION
The results show the differences in quantitative DTI parameters of skeletal muscle with varying degrees of fatty replacement due to dystrophic processes.
Mixed effect models assessing the association between muscle fat fraction and ADC and FA showed that this association was not significantly impacted by the use of a custom or a selective ROI localization. This finding strengthens the suggestion of previous studies4 that tensor metrics of the dystrophic muscle are not only an expression of fatty replacement but could also be influenced by structural changes of the muscle fiber itself.
Limitations include the lack of biopsies to confirm structural changes in cases. Furthermore, the low prevalence of muscular dystrophies rendered the recruitment of cases difficult and resulted in heterogeneity and small size of the study cohort.
CONCLUSION
Diffusion tensor imaging and chemical-shift-encoded water-fat MRI provide non-invasive insights on muscle fiber architecture in physiological and dystrophic conditions. The effect of MFF on tensor-metrics on ADC and FA is not significantly influenced by technique of ROI-localization, suggesting that change in water diffusion is at least partly influenced by different processes.Acknowledgements
No acknowledgement found.References
1. Galban CJ, Maderwald S, Uffmann K, Ladd ME. A diffusion tensor imaging analysis of gender differences in water diffusivity within human skeletal muscle. NMR Biomed. 2005;18(8):489-98.
2. Saotome T, Sekino M, Eto F, Ueno S. Evaluation of diffusional anisotropy and microscopic structure in skeletal muscles using magnetic resonance. Magn Reson Imaging. 2006;24(1):19-25.
3. Zaraiskaya T, Kumbhare D, Noseworthy MD. Diffusion tensor imaging in evaluation of human skeletal muscle injury. J Magn Reson Imaging. 2006;24(2):402-8. .
4. Ponrartana S, Andrade KE, Wren TA, Ramos-Platt L, Hu HH, Bluml S et al. Repeatability of chemical-shift-encoded water-fat MRI and diffusion-tensor imaging in lower extremity muscles in children. AJR Am J Roentgenol. 2014;202(6):W567-73.
5. Damon BM, Ding Z, Anderson AW, Freyer AS, Gore JC. Validation of diffusion tensor MRI-based muscle fiber tracking. Magn Reson Med. 2002;48(1):97-104.
6. Mori S, van Zijl PC. Fiber tracking: principles and strategies - a technical review. NMR Biomed. 2002;15(7-8):468-80.
7. Froeling M, Nederveen AJ, Nicolay K, Strijkers GJ. DTI of human skeletal muscle: the effects of diffusion encoding parameters, signal-to-noise ratio and T2 on tensor indices and fiber tracts. NMR in biomedicine. 2013;26(11):1339-52.
Figures