5047
T1ρ dispersion assessment of articular cartilage in a rabbit ACL transection model1Research Unit of Medical Imaging, Physics and Technology, University of Oulu, Oulu, Finland, 2Medical Research Center, University of Oulu and Oulu University Hospital, Oulu, Finland, 3Department of Applied Physics, University of Eastern Finland, Kuopio, Finland, 4Human Performance Laboratory, Faculty of Kinesiology, University of Calgary, Calgary, AB, Canada, 5Department of Diagnostic Radiology, Oulu University Hospital, Oulu, Finland
Synopsis
T1ρ dispersion measurements were performed to assess early degenerative changes in articular cartilage in an experimentally induced anterior cruciate ligament transection (ACLT) rabbit model. T2 and T1ρ with spin-lock field amplitudes of 500 Hz, 1 kHz and 2 kHz were measured at 9.4 T. Cartilage mechanical properties and proteoglycan content were determined using indentation testing and quantitative histology, respectively. T1ρ dispersion identified degenerative alterations in cartilage as early as two weeks after ACL transection. The dispersion parameters were statistically correlated with biomechanical properties and proteoglycan content.
Introduction
T1ρ dispersion is characterized by the dependence of the T1ρ relaxation time on the strength of the spin-locking field amplitude1.It has been previously correlated to mechanical properties2 and to proteoglycan content3 in articular cartilage. The objective of this study was to investigate the characteristics of T1ρ dispersion, and its relationship with degenerative changes, biomechanical properties and proteoglycan content in a post-traumatic model of osteoarthritis.Methods
Unilateral anterior cruciate ligament transection (ACLT) was performed in 14 skeletally mature New Zealand White rabbits. Animals were sacrificed two (n=8) and eight weeks (n=6) post-surgery, and the femoral condyles and tibial plateaus were harvested from the ACLT and intact contralateral (CL) knee joints. An additional 16 non-operated rabbits served as control (CTRL). Dispersion measurements were performed on a 9.4 T MRI scanner using a T1ρ preparation block consisting of spin-lock (SL) pulses (SL times (TSLs) = 0, 4, 8, 16, 32, 64 and 128 ms) embedded between adiabatic half passage pulses; coupled to a fast spin echo readout (TR = 5 s, matrix = 256x128, slice thickness = 1 mm and FOV = 18x18 mm). T1ρ experiments were repeated for SL frequencies (γB1 = 500, 1000 and 2000 Hz). T2 relaxation time measurements, which approximate the T1ρ at SL frequency of 0 Hz4, were performed with the same experimental setup (TE = 8.7, 12.6, 18.2, 26.4, 38.2, 55.3 and 80 ms).
Relaxation maps were calculated using MATLAB. Means and confidence intervals (CIs) of the relaxation parameters were determined on 2-mm wide full-thickness regions of interest (ROIs), including the load bearing biomechanical testing sites of the lateral and medial femoral condyles and tibial plateaus. Group differences were determined using Kruskal-Wallis testing. Biomechanical properties of the specimens were determined using indentation testing with a plane-ended indenter (diameter = 1 mm). A 3-step stress-relaxation (15 min relaxation time) and sinusoidal dynamic tests (frequency = 1 Hz) were performed to define the equilibrium (Eeq) and dynamic (Edyn) elastic moduli, respectively. Safranin-O optical density (OD) microscopy was performed to quantify tissue proteoglycan (PG) content5. Pearson’s correlations between relaxation parameters (full-thickness ROIs), biomechanical properties, and OD were determined after pooling all femoral cartilage specimens from CTRL, CL and ACLT groups.
Results
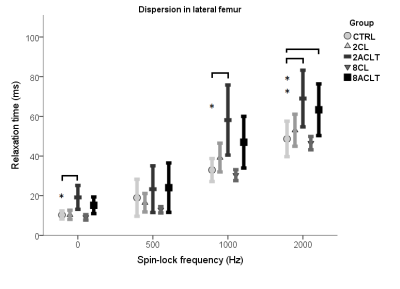
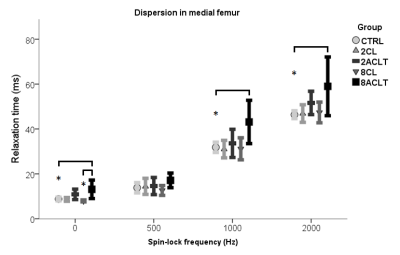
Compared to control knees, cartilage T2 and T1ρ at SL frequencies of 1 kHz and 2 kHz were significantly elevated 2 weeks after ACLT (Fig. 1). Significantly prolonged T2 and T1ρ at SL frequencies of 1 kHz and 2 kHz in the medial, and T1ρ at SL frequency of 2 kHz in the lateral femur were observed 8 weeks after ACLT (Fig. 1-2). Compared to the contralateral knee samples, T2 mean values were significantly longer in the medial compartment at 8 weeks following ACLT (Fig. 2). No statistical significance was found between contralateral and control samples from the femoral condyles and between all groups in the tibial plateau samples.
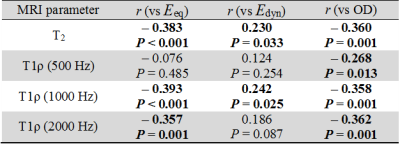
Pooling the lateral and femoral condyle specimens, T2 and T1ρ relaxation parameters were significantly correlated with biomechanical properties and OD (Table 1). Specifically, the equilibrium and dynamic moduli correlated best with T1ρ at an SL frequency of 1 kHz (r = -0.393, p < 0.001; r = 0.242, p < 0.05, respectively) and for OD with T1ρ at an SL frequency of 2 kHz (r = -0.362, p < 0.01).
Discussion
T2 and T1ρ dispersion changed with degenerative changes in cartilage as early as 2 weeks after ACLT. Modulating the SL field amplitude changed the sensitivity of T1ρ to cartilage degeneration at 8 weeks following ACLT. No SL field amplitude demonstrated superior sensitivity to early degeneration. T1ρ at different SL field amplitudes behaved differently with post-traumatic alterations, biomechanical properties and histology. Based on the current findings, an SL amplitude of 500 Hz, mostly used in clinical settings2, was the least sensitive frequency in detecting early structural alterations in cartilage. Using contralateral joints as controls6 remains controversial.
Consistent with previous studies2,3,6,7, T1ρ dispersion was associated with changes in biomechanical properties and optical density (reflective of PG content). Previous studies demonstrated T1ρ correlations with biomechanical properties and PG content at SL field amplitudes of 500 Hz and 1 kHz. In this study, the SL field amplitude was extended to 2 kHz, which was found to be more strongly correlated with PG content than lower SL fields, most likely because of the increased contribution of chemical exchanges to the relaxation properties with increasing SL frequency.
Conclusions
T1ρ analyses at various spin-locking field amplitudes can reveal degenerative changes in articular cartilage as early as 2 weeks post-traumatic intervention, and is associated with biomechanical properties and macromolecular content.Acknowledgements
Support from the Academy of Finland (grants #285909 and #293970), and Jane and Aatos Erkko Foundation is gratefully acknowledged.References
1. Sepponen et al. J Comput Assist Tomogr 9:1007-11, 1985.
2. Keenan et al. Cartilage 6:113-122, 2015.
3. Duvvuri et al. Magn Resonan Med 38:863-67, 1997.
4. Askella et al. Magn Reson Med 52:1103-9, 2004.
5. Kirali et al. Histochem 28:99-107, 1996.
6. Rautiainen et al. OsteoArth Cart 22(10):1444-1452, 2014.
7. Li et al. Magn Reson Imaging 29:324-34, 2011.
Figures