5030
R2-Star & DCE-Perfusion MRI based Novel Approach for Classification of Intra Tumoral Susceptibility Signal (ITSS) into Haemorrhage, Non-Leaky Vessels and Leaky Vessels in Glioblastoma1Centre for Biomedical Engineering, Indian Institute of Technology, Delhi, New Delhi, India, 2Biomedical Engineering, AIIMS, New Delhi, Delhi, India, 3Philips Health Systems, Philips India Limited, Gurgaon, India, 4Department of Radiology and Imaging, Fortis Memorial Research Institute, Gurgaon, India, 5SRL Diagnostics, Fortis Memorial Research Institute, Gurgaon, India
Synopsis
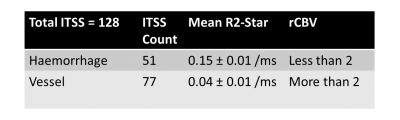
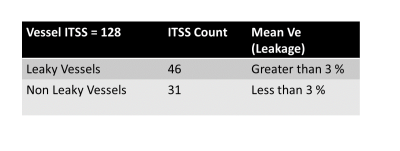
Susceptibility-weighted-imaging (SWI) demonstrates intra-tumoral-susceptibility-signal (ITSS) which could be a combination of haemorrhage and vasculature. True biological classification is necessary to understand the tumor-viability, aggressiveness and angiogenesis. This study develops a novel quantitative approach which combines SWI, R2-Star-relaxivity and DCE-MRI parameters for segmenting ITSS and its further classification into biological-behavior-based sub-categories. After analysis of 128 ITSS from 25 high-grade-glioblastoma patients, we found haemorrhages have higher R2-Star and lower rCBV values compared-to vessel ITSS. Leakage parameter Ve from tracer-kinetic analysis is found as differentiator between leaky and non-leaky-vessels. Proposed approach enables automatic-classification of ITSS into haemorrhage, non-leaky (passive) and leaky (aggressive) vessels.
Purpose
In high-grade glioblastoma, using Susceptibility weighted imaging (SWI) intra-tumoral-susceptibility-signal (ITSS) can be segmented which can be vascular structures as well as haemorrhage. Works have been reported on count of ITSS and its correlation with dynamic-susceptibility-perfusion (DSC) parameters to help tumor grading1-2. However, true biological classification of ITSS is challenging but need-of-the-hour to differentiate haemorrhage and analyze types of vasculature to better understand the tumor-viability, aggressiveness and angiogenesis. Development of automatic methods is required for further classification of ITSS. Dynamic-contrast-enhanced (DCE) perfusion promises to be a better method as it provides both tracer-kinetic3-4 and hemodynamic parameters. This study develops a novel quantitative approach which combines SWI, R2-Star and DCE-MRI parameters for segmenting ITSS and its further classification into biological-behavior-based sub-categories. We hypothesize that based on R2-Star relaxivity values and hemodynamic characteristics, haemorrhage and vessels can be differentiated. We further hypothesize, leaky (aggressive) and non-leaky (passive) vessels can be differentiated using leakage parameters from tracer-kinetic analysis.Method
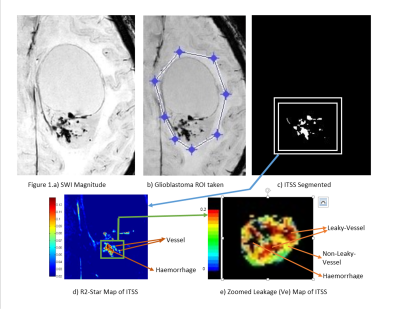
This IRB approved retrospective study included total 25 histology-confirmed glioblastoma patients. All patients underwent conventional MRI, SWI and DCE-MRI (Ingenia-3.0T, Philips Healthcare, The Netherlands). SWI was acquired with 4 echoes at 5.6,11.8,18 and 24.2 ms (slice thickness 1-mm, FOV 240×240 mm2, matrix 384×384). The SW-Magnitude images are obtained from scanner by multiplying FFE-M image with a phase mask derived from PADRE (Phase-Difference-Enhanced-imaging)5 algorithm. DCE-MRI (TR/TE=4.4/2.1ms, FOV 240 × 240 mm2, matrix 128×128, 12 slice with thickness 6-mm, dynamic 32 with temporality 3.9s, contrast dose 0.1 mmol/kg body weight, 3.5 ml/sec injection rate, contrast used Gd-BOPTA). Perfusion (CBF, CBV) and kinetic parameters (Kep, Ktrans, Ve and Leakage) were estimated from DCE-MRI data5,6 and R2-Star maps were generated from the multi-echo SWI magnitude images7 using in-house developed software. All the maps were co-registered with respect to conventional T1W image. Thresholding and shape-based structuring element dependent algorithm developed in MATLAB was used to segment ITSS from SWI-magnitude images. Total 25 patients contributed to a sum of 128 such ITSS segmented for further analysis. R2-Star and DCE-kinetic parameter (Kep, Ktrans, Ve and Leakage) values were recorded for each segmented ITSS. Relative Cerebral Blood Volume (rCBV) were obtained by placing same ITSS ROIs on the contralateral side normal brain parenchyma. For determining threshold values, clearly visible haemorrhage and vessels were manually identified by experienced Radiologists based on the shape and structures. All the parametric values (R2-Star, kinetic and hemodynamic) were computed for manually identified haemorrhage and vessels. R2-Star and rCBV of ITSS were used for automatic classification of ITSS into haemorrhage and vessel. Once differentiated as vessel, Mean±SD value of Ve obtained from normal brain parenchyma was used as threshold to segment non-leaky vessels and remaining as leaky vessels.Results and Discussion
R2-Star and rCBV are significantly (p<0.01) differentiating ITSS into Haemorrhage vs vessel, which proved our first hypothesis. Ve value in normal parenchyma is quite small (0.03) and we expect similar value in non-leaky vessel. This threshold resulted in further classification of vessel into leaky vs non-leaky vessels as shown in Table-2. Figure-1 demonstrates one such sample glioblastoma ITSS classification results. Haemorrhages and vascular structures both have high susceptibility effect so both manifest in SWI-M images as hypo-intensities and can be segmented as ITSS. Vessels are linear or dot-like structures (consistent through multiple slices). Haemorrhages are mainly conglomerations containing deoxyhemoglobin and hemosiderin. These cause high susceptibility effects, which leads to fast signal decay and hence high R2-Star values in haemorrhages compared to vessels. rCBV is also found to vary significantly between haemorrhage and vessels. Ideally for haemorrhages, we expected rCBV to be zero. However, the DCE-Perfusion slice-thickness was 6-mm and of low-planar resolution compared to SWI images which contributes to partial-volume-effect for rCBV thus resulting in non-zero values in haemorrhage. Based upon DCE-perfusion imaging at current spatial resolution, we can comment only on the leaky-ness of the macro-vasculature-ITSS. Disruption or lack of a blood-brain-barrier results in leakage of contrast medium into the extravascular extracellular space. Kinetic parameter Ve is a measure of leakage and results show it is a differentiator between leaky and non-leaky vessel.Conclusion
Proposed approach based upon R2-star and DCE-perfusion parameters enabled automatic classification of ITSS into haemorrhage, non-leaky (passive) and leaky (aggressive) vessels. This classification of ITSS might improve diagnosis and grading of glioblastoma.Acknowledgements
The Authors acknowledge technical support of Philips India Limited and Fortis Memorial Research institute Gurugram in MRI data acquisition. Authors thank Dr. Indrajit Saha, Prof. RKS Rathore, Dr. Prativa Sahoo for technical support in data-processing. This work was supported by grant from Science and Engineering Research Board (IN) (YSS/2014/000092).References
[1] Pinker K, Noebauer-Huhmann IM, Stavrou I, et al. High-resolution contrast-enhanced, susceptibility-weighted MR imaging at 3T in patients with brain tumors: correlation with positron-emission tomography and histopathologic findings. AJNR Am J Neuroradiol 2007;28:1280–86
[2] Park MJ, Kim HS, Jahng GH, et al. Semiquantitative assessment of intratumoral susceptibility signals using non-contrast-enhanced high-field high-resolution susceptibility-weighted imaging in patients with gliomas: comparison with MR perfusion imaging. AJNR Am J Neuroradiol Aug 2009: 30:1402– 08
[3] Sahoo P, Rathore RK, Awasthi R, Roy B, Verma S, Rathore D, et al. Subcompartmentalization of extracellular extravascular space (EES) into permeability and leaky space with local arterial input function (AIF) results in improved discrimination between high- and low-grade glioma using dynamic contrast-enhanced (DCE) MRI. J Magn Reson Imaging 2013;38:677-88.
[4] Tofts P, Kermode A. Measurement of the blood‐brain barrier permeability and leakage space using dynamic MR imaging. 1. Fundamental concepts. Magn. Reson. Med. 1991.
[5] Yoneda T (2009) Triple-layer appearance of human cerebral cortices on phase-difference enhanced imaging using 3D principle of echo shifting with a train of observations (PRESTO) sequence. Proc Int Soc Magn Reson Med 17:27
[6] Singh A, et al. Quantification of physiological and hemodynamic indices using T1 dynamic contrast-enhanced MRI in intracranial mass lesions. J. Magn. Reson. Imaging 2007;26:871–880.
[7] Haacke EM, Cheng NY, House MJ, et al. Imaging iron stores in the brain using magnetic resonance imaging. Magn Reson Imaging 2005; 23: 1–25.