5026
Direct D2O MRI for quantifying tissue permeability: application to Vitreous1Research Imaging Institute, University of Texas Health at San Antonio, San Antonio, TX, United States, 2University of Texas Health at San Antonio, San Antonio, TX, United States
Synopsis
D2O is a freely diffusible trace for calculating blood flow and give more accurate than limited diffused Gadolinium based contrast agents. A few publications from over twenty years ago used MRI or single-voxel NMR spectroscopy to assess D2O (or 17O water) for tissue perfusion. However, these studies never investigated the application of D2O MRI to altered permeability. Our current study explore the dynamic direct D2O MRI to map tissue permeability, and applied it to map the water exchange in rodent vitreous.
Introduction
Deuterium oxide (D2O) has been used as a contrast for MRI a long time due to its efficient signal, low toxicity and commercial availability. It has been used in mapping tissue blood flow1, and also found to be capable of mapping fluid dynamics. In the current study, we aim to quantify ocular water permeability using D2O MRI with submillimeter resolution, and explore its application to map the fluid exchange into the vitreous. Dynamic deuterium MRI was acquired in mouse eye with bolus injection using bSSFP calibrated with reference samples. We further explore different pharmacokinetic models (classical tofts, and dual-input 2-compartment exchange model (2CXM) in estimation of D2O permeability and potential extracellular compartment between vitreous and retinal vasculature2.Method
We scanned anesthetized (1.5% isoflorane) C57BL/6J mice (n=3, 31-36g) with double a D2O / 1H2O surface coil at 7T (Bruker). Four IV boluses of 5ml/kg of D2O (with 0.9% sodium chloride solution) was administrated through cannulated lateral tail vein MRI used the following parameters, 0.4*0.4*1.5 mm resolution, bSSFP acquisition with TE/TR=4/8ms, field of view (FOV) = 25.6x25.6x24mm, matrix=64x64x16, and 5 averages giving temporal resolution of 41.6s. D2O MRI signal was normalized by summing the whole vitreous signal, normalizing by average mouse vitreous volume, then normalizing by two D2O phantoms of known concertation placed on either side of the eye to give % D2O concentration by volume of the vitreous. Raw Arterial input function of D2O was estimated from published rat arterial input function of 15O-water with same rout of administration. 3 Unit of radioactivity in AIF was converted into % mL water injected/mL total blood volume, and adjusted the variation of body weight, blood volume (estimated from body weight), and injection dose between the studies. AIF after 2 min (due to the half-life of 15O tracer technique) was extrapolated using bi-exponential fitting. Mean % D2O tissue uptake curve for each repeated bolus was normalized by subtracting baseline signal before injection. Both AIF and mean % D2O tissue uptake curve were interpolated into same time frame. As vitreous is avascular, classic Tofts model was used for calculating permeability, comparing with results in 2CXM model that potentially separate flow and permeability effect.Result
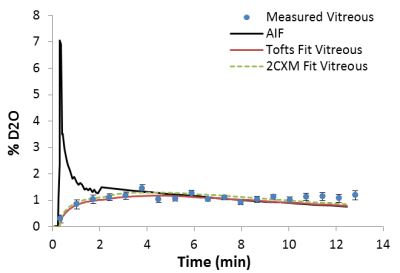
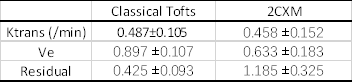
After rescaling, the AIF has peak value around 6.9 and ~1.2 %D2O at the end of washout period, which agree with the D2O injection dose (~6.8 %ml) and the tissue uptake in steady state. Figure1 shows the mean tissue uptake, AIF and the fitted model curve, (pixelwise result is feasible but homogenous in our case). Calculated Ktrans is consistent using different models. Values are reported in the Figure2. Additionally, 2CXM gives Vp 0.328 ±0.121, fp 0.640 ±0.172 indicating its highly perfused. High Ve value in both model imply high extravascular space of the vitreous as expected.Discussion
Originally D2O MRI has been proposed for perfusion imaging years ago, with limited resolution and long acquisition time. The method we proposed shows that D2O has potential to map the water exchange and permeability in vivo. Our result reconfirms that the D2O (mainly in the form of HOD in vivo) is a nearly free diffusing agent and highly diffused into viterous in healthy condition, with Ve close to 1 in different type of fitting. The Ktrans we calculated are significantly higher than Ktrans reported using Gd DCE-MRI3, which is supposed to be low in intact Blood-Retina-Barrier. The 2CXM model separating the weak blood flow and permeability components seems to work not as good as classic Tofts model, which is mathematically equivalent to Kety-Schmidt equation used for early D2O NMR analysis and 15O water perfusion studies2. Due to the mathematical similarities between Tofts model and the classic Kety equation used in earlier D2O NMR studies, we found the water exchange rate reported in venturous5 is in agreement with our speculation4.Further data would need to validate the accuracy of direct D2O method against 15O PET method, and indirect D2O MRI recently published. Advanced model for pure water exchange using different dose of D2O would give potential to mapping the fluid exchange function in vitreous.Conclusion
In the current study, we establish the feasibility to model permeability using dynamic direct D2O MRI. D2O is more freely diffused that conventional Gd-based contrast agent, and have great potential in mapping permeability and water exchange in normal tissue in human.Acknowledgements
The author would like to thank Amor B. and Loddie Lee Whitehead Fellowship Fund in Ophthalmic Research, and Ng, Samuel Barnes for sharing DCE-MRI processing scripts on Github.References
1 J.J.H. Ackerman, C.S. Ewy, N.N. Becker, R.A. Shalwitz, "Deuterium Nuclear-Magnetic-Resonance Measurements of Blood-Flow and Tissue Perfusion Employing (H2o)-H-2 as a Freely Diffusible Tracer," P Natl Acad Sci USA 84, 4099-4102 (1987).
2 S.P. Sourbron, D.L. Buckley, "On the scope and interpretation of the Tofts models for DCE-MRI," Magn Reson Med 66, 735-745 (2011).
3 N. Kudomi, H. Sipila, A. Autio, V. Oikonen, H. Liljenback, M. Tarkia, J. Laivola, J. Johansson, M. Teras, A. Roivainen, "Cross-validation of input functions obtained by H(2) 15O PET imaging of rat heart and a blood flow-through detector," Mol Imaging Biol 14, 509-516 (2012).
4 J.H. Kim, G.H. Im, J. Yoon, J. Yang, J.J. Chung, J.H. Cha, S.I. Kim, D.I. Ham, J. Hee Lee, "Dynamic contrast-enhanced MRI for assessing therapeutic response of choroidal neovascularization in a rat model," Invest Ophthalmol Vis Sci 53, 7693-7700 (2012).
5 T. Obata, H. Ikehira, F. Shishido, N. Fukuda, Y. Ueshima, M. Koga, H. Kato, F. Kimura, Y. Tateno, "Deuterium MR in vivo imaging of the rat eye using 2H2O," Acta Radiol 36, 552-555 (1995).