5025
High rotating frame relaxation MRI mapping for detecting ischemia in rats1Brain repair and rehabilitation, University College of London, London, United Kingdom, 2Center for Magnetic Resonance Research, University of Minnesota, Minneapolis, MN, United States, 3Diagnostic Imaging Canter, Kuopio University Hospital, Kuopio, Finland, 4Brain Repair and Rehabilitation, University College of London, London, United Kingdom
Synopsis
The goal of this work is to evaluate novel MRI technique entitled Relaxation Along a Fictitious Field (RAFF) in the rotating frame of rank n (RAFFn, with n=4) in its ability to detect ischemia, specifically when tuning the sensitivity of RAFF4 to exchange processes of amide protons. Relaxation maps were thus measured in healthy and infarcted rats at 24h post stroke, and were compared with other conventionally used quantitative MRI modalities. RAFF4 robustly detected infarcted regions with enhanced contrast as compared to T1 relaxation and MTR asymmetry, thus offering a sensitive novel MRI marker for quantifying tissue abnormalities during ischemia.
Introduction
Ischemic stroke is one of the most frequent causes of severe disability and death during which the brain undergoes a complex cascade of physiological and metabolic disturbances.The goal of this work it to evaluate novel MRI technique entitled Relaxation Along a Fictitious Field (RAFF) in the rotating frame of rank n (RAFFn) (1), (2) in its ability to detect ischemia when specifically tuning the sensitivity of RAFF4 to exchange of functional groups. Here we demonstrate by theoretical simulations that exchange-induced relaxations during RAFFn (n=4) are accelerated by tuning the periodicity of irradiation to the chemical shifts of interest through generation of side bands. Because the range of exchange rates in vivo seems to be dominated by slow base-catalyzed rates corresponding to amide protons (3), the frequency-modulated part of the RAFF4 pulse was tuned to the exchange rate of amide protons (≈30 Hz). RAFF4 relaxation maps were measured in healthy and infarcted rats at 24h post stroke. We hypothesise that RAFF4 imaging enhances imaging specificity to ischemic acidification during stroke because of the reduced amide exchange rate. A comparison with conventional techniques, including free precession T1 and T2 relaxation mapping, magnetization transfer ration (MTR) asymmetry and APT* is also presented.Methods
Five male Sprague-Dawley (SD) rats (weighing 200-250g) were used for MRI experiments. A middle cerebral artery occlusion was performed as described previously (4). Animals were anaesthetized with isofluorane (3% for induction and 2% during scanning) via a vaporizer with a mixture of air. During the scanning the body temperature was maintained at 37°C with a heating pad, while respiration rate was constantly monitored using MR compatible devices.
Animals were scanned at baseline and at 24 hours post stroke on a 9.4 T Agilent MRI scanner. Images were acquired in a single slice (thickness=2mm), centred on thalamus. Chemical Exchange Saturation Transfer (CEST) measurements were acquired using a gradient-echo sequence (matrix: 64x64, TR=2.11ms, TE=1.07ms, FOV=20x20mm²) with a saturation train of Gaussian pulses at 0.6μT (n=80, pulse duration=50ms, flip angle=360°, 90% duty cycle). Saturation was applied at 71 frequency offsets between -5.0 and 5.0ppm. MTR asymmetry and APT* were generated as described previously (5).
T2 - weighted images were collected using Multiple Spin Echo (MSE) pulse sequence consisting of a 90° Sinc excitation pulse along the x axis (duration=2ms) followed by 10 Sinc refocusing pulses along the y axis (duration=1.6ms). Other parameters were as follows: TR=3s, time interval between the peaks of the pulses τ=8.33ms, slice thickness=2mm, FOV=20x20mm², matrix size=64x64.
An inversion recovery EPI sequence was used to quantify the T1 values. A global adiabatic inversion pulse (duration=2ms) was applied followed by 10 inversion times exponentially spaced from 8.1ms to 7.5s. Other parameters were as follows: TR=15s, TE=25.5ms, slice thickness=2mm, FOV=20x20mm², matrix size=64x64.
RAFF4 measurements were taken using a train of RAFF4 pulses (0, 20, 50, 100) with pulse parameters omega1=244 Hz and pulse duration Tp=4.525ms followed by a single shot spin echo EPI read out with parameters: TR=3s, TE=25.5ms, slice thickness=2mm, FOV=20x20mm², matrix size=64x64.
The simulations were conducted using full set of two-pool Bloch-McConnell equations, as described in (2). Spin populations of the pools were set to Pa=0.1 and Pb =1-Pa and tex between the pools of 10ms. Simulation of evolution of magnetization was done in Matlab by solving Bloch McConnell equations by numerical partial differential equation solver.
Results
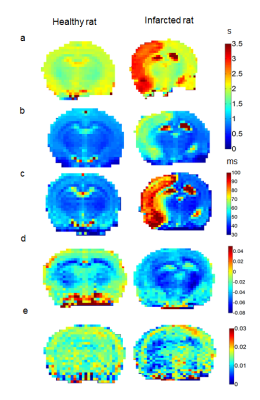
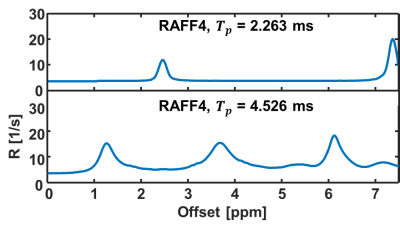
Figure 1 displays the T1, T2, TRAFF4, MTRasym and APT* at 3.5 ppm. Table 1 shows the mean std of imaging parameter in (Stroke lesion - Contralateral region)/ Contralateral region) * 100% for all the maps listed above. Figure 2 shows simulated data using RAFF4 pulses. By changing the pulse duration from 4.5 msec to 2.26 msec RAFF pulse are no longer sensitive to probe amide exchange as shown in Figure 2 (a).Discussion
In this study we demonstrate that RAFF4 contrast enables enhanced sensitivity to detect ischemia as compared to T1, MTRasym analysis and APT*. The frequency-modulation part of RAFF4 pulses was here tuned specifically to exchange of amide protons. Interestingly, sensitivity similar to RAFF4 was detected with T2 mapping. This observation is attributed to sufficiently large repetition rate of the MSE sequence which allowed generating rotating frame T1r weighting. Our results demonstrate significant increase in T1, T2, TRAFF4 during stroke, accompanied by a reduction in APT* and MTR asymmetry maps. These findings are likely related to tissue acidification, cell death, imbalance in glucose/oxygen delivery and consumption during stroke leading to anaerobic metabolism and mitochondrial poisoning (3). Future studies, both in vivo and in vitro, are underway for further characterization of the RAFFn contrast upon variations of the positions of sidebands which are supposed to sensitize the contrast to different exchange processes.Acknowledgements
We gratefully acknowledge our funders: NIH P41 EB015894,Instrumentarium Science Foundation (HL, Finland), Department of Health's NIHR Biomedical Research Centre UCL/UCLH.References
1. Liimatainen T, Hakkarainen H, Mangia S, Huttunen JMJ, Storino C, Idiyatullin D, et al. MRI contrasts in high rank rotating frames. Magn Reson Med. 2015 Jan;73(1):254–262.
2. Liimatainen T, Mangia S, Ling W, Ellermann J, Sorce DJ, Garwood M, et al. Relaxation dispersion in MRI induced by fictitious magnetic fields. J Magn Reson San Diego Calif 1997. 2011 Apr;209(2):269–76.
3. Zhou J, Payen J-F, Wilson DA, Traystman RJ, Van Zijl PCM. Using the amide proton signals of intracellular proteins and peptides to detect pH effects in MRI. Nat Med. 2003;9(8):1085–1090.
4. Kiozumi J, Yoshida Y, Nakazawa T, Ooneda G. Experimental studies of ischemic brain edema: I: A new experimental model of cerebral embolism in rats in which recirculation can be introduced in the ischemic area. Jpn J Stroke. 1986;8:1–8.
5. Jin T, Wang P, Zong X, Kim S-G. MR imaging of the Amide-Proton Transfer effect and the pH-insensitive Nuclear Overhauser Effect at 9.4 T. Magn Reson Med Off J Soc Magn Reson Med Soc Magn Reson Med. 2013 Mar;69(3):760–770.
Figures