5021
Nanomicelles – A Blood Pool Contrast Agent for MRI1School of Pharmaceutical Sciences, University of Geneva, Geneva, Switzerland, 2Division of Radiology, Geneva University Hospitals, Geneva, Switzerland, 3Institut Translationnel d'Imagerie Moléculaire, University of Geneva, Geneva, Switzerland, 4Service Développement et Croissance, University of Geneva, Geneva, Switzerland, 5Laboratoire d'Imagerie Fonctionnelle et Métabolique, Ecole Polytechnique Fédérale de Lausanne, Lausanne, Switzerland, 6Group of Inorganic and Bioinorganic Chemistry, Ecole Polytechnique Fédérale de Lausanne, Lausanne, Switzerland
Synopsis
A blood pool MRI contrast agent for free breathing MR angiography is highly desirable in particular for pediatric cardiovascular exams in order to improve both image quality and patient comfort. Apnea in infants and be propofol induced, or via intubation, which is invasive and limits acquisition to short protocols. We present here initial in-vivo studies in mice of a newly developed nanomicelles gadolinium contrast agent. Persistent vascular enhancement is observed with no toxicity. Micelles also open the way to functionalization for theranostic applications.
Background
Contrast agents (CA) are already commonly used in cardiovascular MRI, particularly for angiography (MRA). In pediatric cardiovascular MRI, blood pool agents would allow the realization of MRA during free breathing with light anesthesia while extra-vascular contrast agents currently used (e.g. Dotarem®, gadoteric acid, Gd-DOTA) require apnea to avoid artifacts related respiratory movements. In this patient population, this requires anesthesia with intubation or propofol apnea, which is not well tolerated or leads to a shortened protocol. A blood pool agent would also be highly advantageous in 3D cine MRI applications to provide blood/myocardial contrast over a longer acquisition times with relatively low dose1.
Such ‘blood pool agents’ exist, i.e. Ablavar®, (gadofosveset trisodium) which has the advantage of a large intravascular persistence due to interaction with blood proteins with a primarily renal extraction2. This agent was recently withdrawn from the market for licensing reasons.
Our recently synthesized CA should exhibit similar properties to Ablavar®, but with additional advantages of blood persistence due to size, no protein interactions and higher relaxivity due to increased concentration of gadolinium per molecule. The new CA is composed of nanomicelles spontaneously formed in water from amphiphilic building blocks. This also open the potential for functionalized micelles for theranostic purposes.
Methods
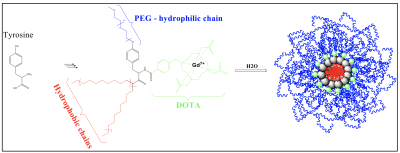
The DOTA-bearing amphiphilic building blocks were synthesized by binding lipophilic chains and a polyethylene moiety on a center tyrosine amino acid. Nanomicelles were obtained by nanoprecipitation. Building blocks (MM=3198Da, 10.1mg) were dissolved in a acetone (200µL) and ethanol (200µL) mixture. The organic phase was added dropwise (syringe fitted with a 21G needle) into ultrapure water (1400µL) over 5min under magnetic stirring. The organic solvents and part of the water were evaporated under reduced pressure in 30min to obtain 0.5g a of final liquid sample. Dilution with PBS to 0.9g gave a final micellar solution at 3.5mM of building blocks. Figure 1 shows structure.
Nanomicelles were characterized by transmission electron microscopy. Size and stability was determined by dynamic light scattering and the relaxivity by NMR. Cell viability was tested via the WST-1 assay on HT1080 cells. Finally, the micelles were imaged by MRI (Mediso nanoscan 3T) in a 96-well plate phantom and compared to commercial contrast agent Gd-DOTA. After full characterization of micelles, mice were injected intravenously in the tail vein with either Gd-DOTA (current standard MRI angio contrast agent, n=3) or nanomicelles (n=3).
MRI used a cryogen-free Mediso nanoscan 3T small animal magnet (10cm-diameter bore) equipped with 525mT/m gradient coils with a birdcage coil of 3 cm diameter for transmission and reception. Mice imaged prior to injection were used as controls. 3D T1-weighted images were acquired twice in the first 30 minutes and subsequently every 30 min up to 3 hours after injection (parameters: FOV=80x40mm, Matrix=256x128, 80 slices, 0.5mm thickness, flip angle=40°, TE/TR=3.8/10ms, 20 averages). A 3D cine triggered on ECG signal was acquired 1 hour after injection (parameters: FOV=40x40mm, Matrix=128x128, 60 slices, 0.3mm thickness, flip angle=30°, TE=4ms, 2 averages, 15 cardiac phases, depending on heart rate, with temporal resolution 8.33ms). Signal intensity was measured in the arteries and major organs, and normalized to muscle signal.
Results
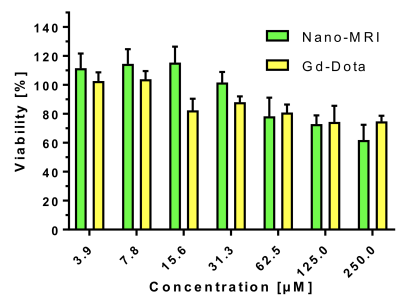
Micelles were stable in aqueous media and had a mean size 10 ± 3 nm. They did not alter cell viability at concentrations used for imaging (fig2). In a phantom, Micelles gave up to 20% higher signal than Gd-DOTA at equivalent concentrations, with a relaxivity of 27 mM-1s-1 at 20MHz.
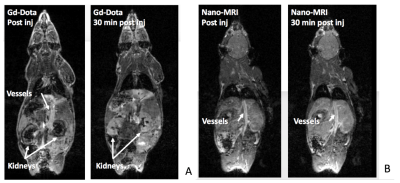
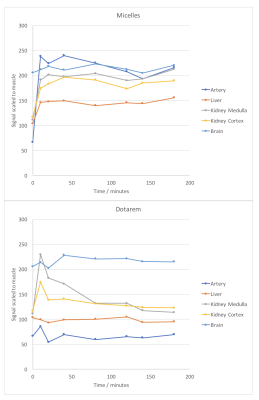
In vivo, Gd-DOTA was rapidly eliminated via the kidneys and bladder (Fig3a), whereas Micelles remained in the blood compartment over the 3 hour study. Micelles significantly enhanced the vessels and the heart for T1W images (Fig3b) and enhanced blood myocardium contrast on cine MRI (Fig4). After intravascular injection, liver and kidney parenchyma signal was likely related to blood pool enhancement in small vessels. Quantification of normalized signal intensity in figure 5 shows the different uptake and elimination pathways.
Discussion
Characteristics of nanomicelles (size and relaxivity) were appropriate for in-vivo preclinical tests. The micelles were stable and non-cytotoxic. The gain in T1W signal observed in a phantom over Dotarem showed potential for a competitive MRI contrast agent. In-vivo, the micelles remain in the vascular compartment for a prolonged period of time compared to Dotarem illustrating the different mode of action and elimination, and the true blood-pool nature of these micelles.Conclusion
In conclusion, this study shows the first promising in-vivo imaging results of this candidate blood pool contrast agent which could improve pediatric cardiovascular imaging in terms of image quality and patient comfort. The micelle structure has potential for future theranostic applications.Acknowledgements
• Institute of Translational Molecular Imaging (ITMI) of UNIGE https://www.unige.ch/medecine/itmi/institute-of-translational-molecular-imaging-itmi/
• Zootechnie of CMU, UNIGE http://www.medecine.unige.ch/zootechnie/
• Bioimaging Center UNIGE http://bioimaging.unige.ch/
• RS2D/Mediso, especially Marie-Anais Petit
References
(1) Crowe, L. A.; Montecucco, F.; Carbone, F.; Friedli, I.; Hachulla, A. L.; Braunersreuther, V.; Mach, F.; Vallee, J. P. 4D cardiac imaging at clinical 3.0T provides accurate assessment of murine myocardial function and viability. Magn Reson Imaging 2017, 44, 46-54.
(2) Rapp, J. H.; Wolff, S. D.; Quinn, S. F.; Soto, J. A.; Meranze, S. G.; Muluk, S.; Blebea, J.; Johnson, S. P.; Rofsky, N. M.; Duerinckx, A.; Foster, G. S.; Kent, K. C.; Moneta, G.; Middlebrook, M. R.; Narra, V. R.; Toombs, B. D.; Pollak, J.; Yucel, E. K.; Shamsi, K.; Weisskoff, R. M. Aortoiliac occlusive disease in patients with known or suspected peripheral vascular disease: safety and efficacy of gadofosveset-enhanced MR angiography--multicenter comparative phase III study. Radiology 2005, 236, 71-78.
Figures