5015
An improved blood pool MRI agent with dinuclear structure: characterization and in vivo bio-distributionAlberto Fringuello Mingo1, Francesca La Cava2, Luigi Miragoli1, Enzo Terreno2, Enrico Cappelletti1, Luciano Lattuada1, Sonia Colombo Serra1, Luisa Poggi1, and Fabio Tedoldi1
1Bracco Imaging Spa, Colleretto Giacosa, Italy, 2Università degli Studi di Torino, Torino, Italy
Synopsis
A novel dinuclear gadolinium(III) chelate containing two moieties of DTPA, covalently conjugated to deoxycholic acid is presented. The product was synthesized and characterized in vitro (analysis of relaxometric properties in different media) and in vivo (blood pharmacokinetic and MRI bio-distribution). The complex showed a much higher relaxivity than Gd-DTPA and other dinuclear complexes proposed in literature. Moreover, it displayed a strong interaction with human serum albumin in three binding sites. This property translates in a long blood elimination half time (130min in rats) making the product an optimal blood-pool agent.
INTRODUCTION:
Gadolinium-based contrast agents (GBCAs) are widely used in clinical Magnetic Resonance Imaging (MRI). More than 40 million doses are given worldwide each year [1] and search for new candidates is still active [2-4]. The crucial properties of a GBCA in terms of efficacy is the relaxivity (r1), that is the ability to reduce the longitudinal relaxation time (T1) of surrounding protons. The reduction of the molecular rotational motion, attainable either by increasing the size of the contrast agent (e.g. polymeric structures) or by promoting non-covalent, reversible binding with plasma proteins, is one of the most successful ways employed to increase the relaxivity of a GBCA. In this work both strategies have been used, in fact a dinuclear gadolinium chelate containing two moieties of diethylenetriaminepentaacetic acid (DTPA) (complex-1, Figure1), covalently conjugated to deoxycholic acid is proposed. It is worth to notice that the increment of molecular size not only increases r1 but also assigns to the molecule useful pharmacokinetic properties, lengthening the elimination half time.METHODS:
A full relaxometric characterization was carried out and consisted in: (1) the acquisition of nuclear magnetic resonance dispersion (NMRD) in different media (physiologic solution with and without addition of 35g/L of human serum albumin (HSA), two simulated body fluid mimicking the ionic content and the viscosity of plasma (i-SBF and v-SBF, respectively) and human plasma (HP)); (2) the study of binding affinity to HSA; (3) a transmetallation assay [1]. In addition, in vivo MRI bio-distribution and blood pharmacokinetic were compared with Gd-DTPA (Magnevist®, Bayer) and gadocoletic acid trisodium salt (B22956/1, Bracco Imaging Spa), two well-known complexes that respectively share the same chelating cage and the same deoxycholic residue. MRI study consisted in the acquisition of T1-weighted gradient echo images at 1T post GBCA administration, while pharmacokinetic analysis was performed by collecting blood samples at different times post injection and measuring the gadolinium concentration by relaxometric assay and inductively coupled plasma mass spectroscopy (ICP-MS).RESULTS:
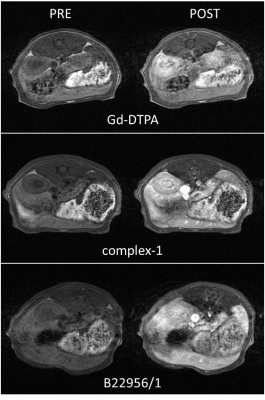
The relaxivity of complex-1 at 20MHz and 37°C in saline is 7.7mM-1s-1; neither the presence of physiological ions nor the viscosity of the medium (up to 1.2mPas) affected significantly r1. Conversely, the addition of HSA to saline as well as the use of plasma medium induced an increase of r1 up to about 20mM-1s-1. NMRD profiles in these two latter media showed the typical peak observed in presence of binding and dedicated titration experiments were performed to assess the affinity constant (KA= 8 103M-1) and the number of binding sites (n=3). The stability of the complex versus transmetallation is comparable to that of the commercial product Gd-DTPA. The in vivo behavior of the complex is shown in Figure2 (representative images before and after administration of the CA in comparison with Gd-DTPA and B22956/1) and Figure3 (percentage signal enhancement as a function of time post injection in different anatomical districts). Blood signal enhancement of Gd-DTPA was about three times lower (despite the double dose) than the remaining CAs, that instead showed comparable values (despite a slightly inferior r1 of complex-1 vs B22956/1 [4]). Complex-1 demonstrated a much slower kinetic in blood than the remaining CAs, also confirmed by the pharmacokinetic study (nearly 7 times longer elimination half time). Complex-1 is excreted both by kidney and liver and slowly accumulates in muscle.DISCUSSION:
The good binding affinity with HSA, the relatively high number of binding sites and the dinuclear structure (which allows to inject half number of molecules at a fixed gadolinium dose) lead to a bound fraction of complex-1 next to 100%. This feature, together with a reduced hepatic elimination compared to B22956/1, gives rise to an extremely long elimination half time, i.e. to unexpectedly strong enhanced properties of blood pooling, confirmed by two completely different techniques (contrast enhanced MRI and relaxometric analysis of blood samples). The in vivo behavior of complex-1 is very similar to a macromolecule, as proven by the slow accumulation over time in muscle, opening the field of application of the complex beyond angiography, since macromolecular CAs are known to preferentially accumulate in tumor tissue.CONCLUSION:
A high relaxivity albumin binder dinuclear gadolinium complex with extraordinarily good confinement in the vascular compartment is proposed as a new candidate for MR angiography and potentially for oncologic application. The macromolecular-like behavior can be in principle smartly exploited to highlight permeability and retention differences between healthy and pathological tissues.Acknowledgements
No acknowledgement found.References
- Parac-Vogt T.N. et al. Chem. Eur. J. 2005;11:3077-3086.
- Longo D. et al. Biomaterials 2016;75:47-57.
- Gianolio E. et al. J Biol Inorg Chem 2014;19:715–726.
- Laurent S. et al. Contrast Media Mol Imaging. 2010;5(6):305-8.