5002
Susceptibility-Weighted Imaging on a Compact 3T Scanner with High-Performance Gradients1Radiology, Mayo Clinic, Rochester, MN, United States
Synopsis
Susceptibility-weighted imaging (SWI) uses a 3D multi-echo gradient-echo sequence with unipolar gradient lobes to acquire all echoes, with fly-back gradients inserted in-between. These fly-back gradients reduce acquisition efficiency and increase echo spacing. We demonstrated that the high gradient performance available on a compact 3T system can reduce the echo spacing in the multi-echo readout by reducing the pulse-width of the fly-back gradient, which consequently allows a greater number of echoes to be sampled. The increased number of echoes translates into reduced noise and improved small vessel conspicuity in SWI acquired on the compact 3T compared to the conventional whole-body system.
INTRODUCTION
Susceptibility-weighted imaging (SWI) has emerged as a useful clinical technique which exploits the susceptibility differences between tissues. It provides the detection of a wide variety of intracranial pathologies that require a high sensitivity to deoxygenated blood, calcium, iron, and small veins [1,2]. SWI usually uses a 3D spoiled gradient-echo sequence, acquiring multiple echoes after each excitation [3]. To ensure phase consistency, unipolar gradients are used to acquire the echoes, requiring the insertion of fly-back gradients between readouts. These fly-back gradients reduce acquisition efficiency and increase spacing between echoes, which consequently reduces the number of echoes that can be sampled in a specified readout period. One way to reduce the echo spacing and accordingly increasing the number of echo train length is to use higher performance gradient to shorten the pulse-width of the fly-back gradients.
A low-cryogen, compact 3T (C3T) MRI system for brain, extremity and infant imaging was recently developed to meet the clinical demands for a light-weight, low-cost and high-performance MR system [4-6]. The C3T is equipped with a high-performance gradient system, which can achieve 80 mT/m gradient amplitude and 700 T/m/s slew rate simultaneously without peripheral nerve stimulation. In comparison, the gradient system on a 60-cm bore whole-body system like a GE MR750 can reach 50 mT/m and 200 T/m/s at its maximum. The high gradient performance on the C3T can particularly benefit acquisitions with a unipolar echo train using fly-back gradients. In this work, we presented the SWI on the C3T exploiting the high performance gradient system to achieve reduced echo spacing and increase the number of sampled echoes in a pre-defined TR.
METHODS
Under an IRB- approved protocol, one healthy volunteer was scanned on both a standard whole-body MR system (GE Discovery MR750, GE Healthcare, Waukesha, WI) and the C3T using an 8-channel receiver coil (Invivo, Gainesville, Florida). Consistent imaging parameters were used, i.e., maintaining the TR, TE, FOVs and imaging matrices between the two exams (Table 1). The C3T used real-time gradient pre-emphasis (7) and frequency shifting to compensate additional concomitant fields (8) due to the gradient asymmetry. The higher gradient performance reduced the echo spacing by 23%, which consequently increases the echo train length from 6 to 8 for nearly equivalent TE and TR times.
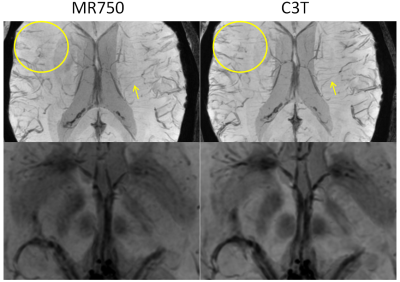
In addition to visual comparison, contrast-to-noise ratio (CNR) estimation were performed on the SWI images. Region-of-interest (ROI) analyses were used to compare CNR between various anatomic structures including globus pallidus (GP), red nucleus (RN), subthalamic nucleus (STN) and the adjacent white matter (WM). The CNR was defined as the difference between the mean values of signals in ROIs of the structure and WM divided by the standard deviation of signal in the WM ROI.
RESULTS
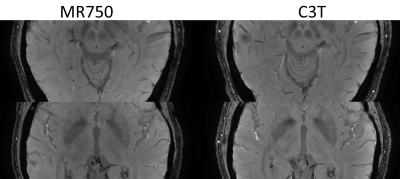
The minimum intensity projections (minIP) through a 20-mm thick sub-region at various axial positions of the brain are shown in Fig. 1. Care was taken to match the anatomical sub-region between the exams on different scanners. Qualitatively, several vessels and deep brain grey matter structures show improved conspicuity in the minIP images acquired on the C3T. SWI acquired on the C3T yielded higher CNR than those acquired on the conventional whole-body scanner as shown in single slice SWI images (Fig. 2). The CNR measurement results are shown in Table 2, which corroborated the observation.DISCUSSION and CONCLUSION
We demonstrated that the high gradient performance available on the compact 3T system can reduce the echo spacing in the unipolar multi-echo readout by reducing the pulse-width of the fly-back gradient, and therefore allow a greater number of echoes to be sampled in a given acquisition time. The increased number of echoes translates into reduced noise and improved small vessel conspicuity. The reduction in ΔTE may also enhance the robustness of the phase unwrapping algorithm to estimate local field map [9]. The improved scan efficiency on the C3T can potentially also be used to reduce the scan time or to improve the image resolution. The work reported is very preliminary, and a more extensive clinical SWI comparison between the two systems is needed to fully demonstrate the potential of the high gradient system for this application.Acknowledgements
This work was supported by NIH grant U01 EB024450-01.References
1. Haacke, E. M., Xu, Y., Cheng, Y.-C. N. and Reichenbach, J. R. (2004), Susceptibility weighted imaging (SWI). Magn. Reson. Med., 52: 612–618.
2. Haacke EM, Mittal S, Wu Z, Neelavalli J, Cheng Y-CN. Susceptibility-weighted imaging: technical aspects and clinical applications, part 1. AJNR Am J Neuroradiol 2009; 30:19-30
3. Duyn J. MR susceptibility imaging. J Magn Reson 2013; 229:198-207.
4. Foo T, Tan ET, Schenck J, Graziani D, Laskaris ET, Vermilyea M, Sabourin C, Shu Y, Huston J, Bernstein MA. Novel High Performance, Compact 3.0T MRI System for Imaging the Brain. Military Health System Research Symposium (MHSRS), Orlando, FLA August 2016.
5. Lee S-K, Mathieu J-B, Graziani D, et al. Peripheral nerve stimulation characteristics of an asymmetric head-only gradient coil compatible with a high-channel-count receiver array. Magn Reson Med 2016;76:1939–1950.
6. Weavers PT, Shu Y, Tao S, Huston J, Lee S-K, Graziani D, et al. Technical Note: Compact three-tesla magnetic resonance imager with high-performance gradients passes ACR image quality and acoustic noise tests. Med Phys 2016;43:1259–64.
7. Tao S, Weavers PT, Trzasko JD, Shu Y, Huston J 3rd, Lee SK, Frigo LM, Bernstein MA. Gradient pre-emphasis to counteract first-order concomitant fields on asymmetric MRI gradient systems. Magn Reson Med. 2017 Jun;77(6):2250-2262.
8. Weavers PT, Tao S, Trzasko JD, Frigo LM, Shu Y, Frick MA, Lee SK, Foo TKF, Bernstein MA. B0 concomitant field compensation for MRI systems employing asymmetric transverse gradient coils. Magn Reson in Med 2017. doi:10.1002/mrm.26790
9. Funai AK, Fessler JA, Yeo DTB, Olafsson VT, Noll DC. Regularized field map estimation in MRI. IEEE transactions on medical imaging. 2008;27(10):1484-1494.
Figures