4980
Longitudinal assessment of TAT-PHP treatment effect in improving brain function recovery from cardiac arrest resuscitation with endogenous perfusion using ASL and metabolic CEST MRI1Campus Center for Neuroimaging, University of California, Irvine, CA, United States, 2Radiology, College of Medicine, University of Illinois, Chicago, IL, United States, 33T Research Program, Center for MR Research, College of Medicine, University of Illinois, Chicago, IL, United States, 4Emergency Medicine, College of Medicine, University of Illinois, Chicago, IL, United States, 5Bioengineering, College of Engineering, University of Illinois, Chicago, IL, United States, 6Research Resource Center, University of Illinois, Chicago, IL, United States, 7Radiology, Northwestern University, Chicago, IL, United States
Synopsis
Sudden cardiac arrest is a leading cause of death in the US. A recently developed peptide-based (TAT-PHP) therapy has been shown to improve survival rate and heart metabolism in animal studies. There is a great need to evaluate the effect of the treatment in the brain as the brain is the most susceptible organ following cardiac arrest. We here demonstrated that the recovery of brain perfusion and metabolism is improved in mice with TAT-PHP treatment vs. controls with endogenous perfusion arterial spin labeling MRI and metabolic MRI based on chemical exchange saturation transfer.
BACKGROUND and PURPOSE
Sudden cardiac arrest (SCA) is a leading cause of death in the US, with an overall survival rate of 7% and brain dysfunction in more than 40% of survivors (1). Post-SCA syndrome includes myocardial dysfunction, brain injury, impaired metabolism, and inflammation. A potential therapy has been developed in recent animal studies, aimed to improve survival rate after SCA (1). The mechanism of the treatment relies on improving cardiac bioenergetic recovery by administrating a peptide (TAT-PHP) responsible for the activation of Akt pathway that promotes cell survival. Despite the promising results, the action of this therapy on the brain tissue is still unclear. In light of the increasing clinical interest on the devastating neurologic injury due to cardiac arrest (2), in this study, we aim to assess the brain perfusion and metabolism recovery after SCA in mice with and without TAT-PHP treatment using endogenous perfusion arterial spin labeling (ASL) MRI and chemical exchange saturation transfer (CEST) MRI.METHODS
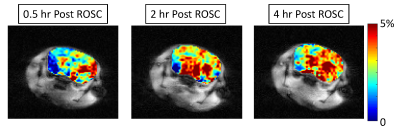
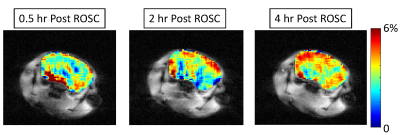
A model of SCA previously established in female C57BL/6 mice was used in this study (1). In brief, mice underwent 8 min of KCl-induced (0.08 mg/g) asystolic arrest followed by cardiopulmonary resuscitation (CPR) including chest compressions, mechanical ventilation, and scheduled fluid administration. TAT-PHP (7.5 mg/kg) was administrated intravenously during CPR. In this preliminary study, two experimental groups were included: 1) saline control (n=4); 2) TAT-PHP (n=2). After mice achieved return of spontaneous circulation (ROSC), they were continuously imaged at a 9.4T animal MRI scanner for up to 4 hours. Repeated MRI experiments included ASL based on signal targeting with alternating radio frequency (STAR) (TR/TE = 2000/9.6 ms, post labeling delay = 500 ms, FOV = 25 x 25 mm2, matrix size = 64 x 64, number of average = 6), B0 mapping based on water saturation shift referencing (WASSR), and CEST MRI (saturation offsets at ±4, ±3.5, ±3, ±2.5, ±2, and +100 ppm, 1 second continuous wave squared saturation pulse of 3.53 µT) (3). For brain perfusion imaging, relative cerebral blood flows (rCBF) were computed as rCBF=100%*(Sctr-Stag)/Sctr. Metabolic CEST contrast at +3 ppm was reconstructed after B0-inhomogeneity correction (4) as CEST+3ppm=100%*(S-3ppm-S+3ppm)/S+100ppm.RESULTS and DISCUSSION
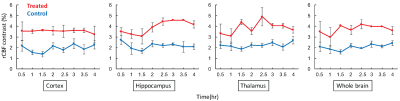
rCBF was consistently higher in mice with TAT-PHP treatment vs. the controls within all brain regions of interest and the whole brain (Fig. 1). Interestingly, rCBF in cortex region was maintained for up to 4 hours, whereas there was a mild and progressive increase in the hippocampus and thalamus regions in the treated mice (Fig. 1-2). The overall increase in rCBF indicates the improvement of cerebral perfusion pressure presumably due to the TAT-PHP treatment. This is in agreement with the previous reports that the TAT-PHP treatment can improves heart function by improving arterial blood pressure recovery (1).
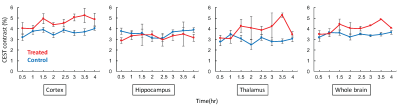
CEST contrast in brain was lower at 0.5 hour after ROSC and appeared to gradually progress over the entire monitoring time course for both groups (Fig. 3). The enhancement of CEST contrast over time likely indicated the recovery in tissue metabolism. Particularly, CEST contrast was higher in treated mice vs controls within cortex, thalamus, and overall in the whole brain regions (Fig. 3), indicating higher concentration of brain metabolites in treated mice. In addition, the rate of CEST contrast enhancement is likely higher in treated mice vs. controls (Fig. 3-4). All these confirmed that TAT-PHP treatment did improve brain metabolism after ROSC.
CONCLUSION
This study demonstrated that TAT-PHP treatment improved brain perfusion as well as brain metabolism recovery measured with dynamic endogenous ASL and CEST MRI. Future research will continue with increased sample size, dose optimization, and clinical translational applications.Acknowledgements
No acknowledgement found.References
1. Li J, Wang H, Zhong Q, Zhu X, Chen SJ, Qian Y, Costakis J, Bunney G, Beiser DG, Leff AR, Lewandowski ED, ÓDonnell JM, Vanden Hoek TL. A novel pharmacological strategy by PTEN inhibition for improving metabolic resuscitation and survival after mouse cardiac arrest. Am J Physiol Heart Circ Physiol. 2015;308(11):H1414-22. Epub 2015/03/20. doi: 10.1152/ajpheart.00748.2014. PubMed PMID: 25795713; PMCID: PMC4451301. 2. Geocadin RG, Koenig MA, Jia X, Stevens RD, Peberdy MA. Management of brain injury after resuscitation from cardiac arrest. Neurologic clinics. 2008;26(2):487-506, ix. doi: 10.1016/j.ncl.2008.03.015. PubMed PMID: 18514823; PMCID: 3074242. 3. Cai K, Singh A, Poptani H, Li W, Yang S, Lu Y, Hariharan H, Zhou XJ, Reddy R. CEST signal at 2 ppm (CEST@2ppm) from Z-spectral fitting correlates with creatine distribution in brain tumor. NMR in Biomedicine. 2015;28(1):1-8. doi: 10.1002/nbm.3216. 4. Cai K, Haris M, Singh A, Kogan F, Greenberg JH, Hariharan H, Detre JA, Reddy R. Magnetic resonance imaging of glutamate. Nat Med. 2012;18(2):302-6. doi: http://www.nature.com/nm/journal/v18/n2/abs/nm.2615.html - supplementary-information.Figures