4979
Fronto-striatal connectivity alterations in a prenatal ethanol exposure rat model: a resting-state functional MRI study1Department of Diagnostic Radiology and Nuclear Medicine, University of Maryland School of Medicine, Baltimore, MD, United States, 2Department of Pediatrics, University of Maryland School of Medicine, Baltimore, MD, United States
Synopsis
People with Fetal Alcohol Spectrum Disorders (FASDs) may present deficits in executive functions. The fronto-striatal circuit is a critical component of the fronto-basal ganglia pathway that plays an important role in executive processes. We used resting-state functional MRI to assess the effects of prenatal alcohol exposure on the functional interaction within the fronto-striatal circuit in a rat FASD model. Male alcohol-exposed rats, but not females, showed reduced fronto-striatal connectivity. Further exploratory analysis revealed a reduction in cortico-striatal connectivity in female alcohol-exposed rats, suggesting that prenatal alcohol exposure has sex dependent effects on executive and sensory processing functions.
Introduction
People with Fetal Alcohol Spectrum Disorders (FASD) often present with deficits in executive functions1. A recent behavioral study found more executive function deficits in male alcohol-exposed rats than in females2. Impaired executive functions are hallmarks of frontal-basal ganglia pathway dysfunction3-5. The fronto-striatal circuit is a critical component of the fronto-basal ganglia pathway where the striatum is the entry point for basal ganglia. Although the fronto-basal ganglia pathway is known to regulate attention, working memory, and executive function3-5, evidence suggests that the caudate nucleus plays a greater role in executive processes compared to other basal ganglia nuclei3,6,7. In this study, we assessed the effects of prenatal alcohol exposure on functional interactions within the fronto-striatal circuit in a rat FASD model. We predict that prenatal alcohol exposure will disrupt functional connectivity within the fronto-striatal circuit, especially in males.Methods
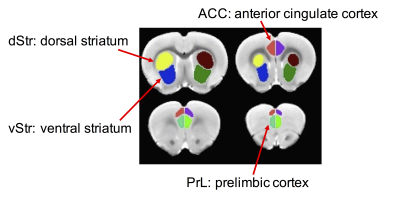
Pregnant Long-Evans rats were fed a diet containing ethanol (Eth) (2.1% v/v ethanol on gestational day (G) 6-G7; 4.27% ethanol on G8-G10; 6.36% G11-G20) or received an ethanol-free control liquid diet (Ctr). 26 Ctr (14 male, 12 female) and 18 Eth (12 male and 6 female) offspring were included in the study. rsfMRI experiment was performed between postnatal day (P)66 and P90 on a BrukerBioSpec 70/30USR Avance III 7T horizontal bore MR scanner (BrukerBiospin MRI GmbH, Germany). Anesthesia was induced with 2% isoflurane followed by intramuscular bolus administration of the dexmedetomidine (0.03 mg/kg) and was maintained using 0.25-0.5 % isoflurane in oxygen enriched air with continuous infusion of dexmedetomidine (0.015 mg/kg/h). An MR compatible small-animal monitoring and gating system (SA Instruments, Inc., New York, USA) was used to monitor the animal respiration rate and body temperature which was maintained at 35–37.5°C. All rsfMRI image processing was conducted using SPM12 (http://www.fil.ion.ucl.ac.uk/spm/) and AFNI (http://afni.nimh.nih.gov/afni). The processing pipeline included slice timing correction, motion correction, alignment to a rat stereotaxic template8, orthogonalization of motion-derived parameters, spike censoring, band-pass filtering and smoothing. The regionally-averaged BOLD time courses were extracted from left and right side of prelimbic cortex (PrL), anterior cingulate cortex (ACC), dorsal and ventral striatum (dStr, vStr) (Fig 1). Functional connectivity was estimated by correlating the time courses between the left and right side of frontal regions (PrL or ACC) and striatal regions (dStr or vStr). Correlation coefficients were transformed to connectivity z-scores with Fisher’s transformation and subjected to a two-way ANOVA analysis with alcohol and sex as two factors. False positive rate (FDR) correction was performed to maintain a 5% type 1 error rate9. Connectivity maps were obtained by correlating the BOLD time course of each ROI with voxels across the whole brain. Two-sample t-test between Ctr and Eth group were performed respectively in males and females. Family-wise error correction was performed with 3dClustSim function in AFNI10.Results and Discussion
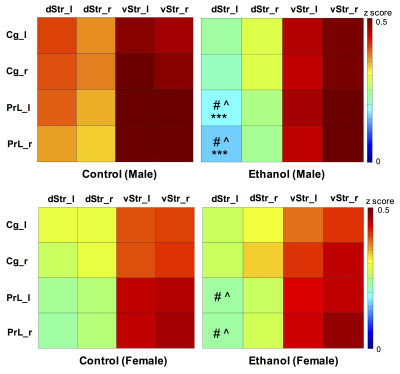
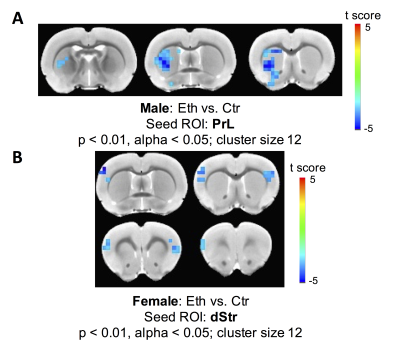
Two-way ANOVA revealed a significant alcohol effect and an alcohol x sex interaction on functional connectivity between PrL and left dStr (FDR corrected). However, the main effect of sex was not significant. One-way ANOVA within males and females showed a reduction of functional connectivity between PrL and left dStr in Eth males but not in Eth females (Fig 2). A significant cluster in left striatum demonstrated reduced connectivity with PrL in Eth males (Fig 3A). Connectivity reduction was observed in vStr of Eth males but to a smaller extent than dStr. No connectivity alteration between other basal-ganglia nuclei and frontal or striatal regions was observed in Eth females. Instead, exploration of alcohol effects on females revealed reduced functional connectivity between dStr and somatosensory cortex (Fig 3B). Striatum is involved in sensory processing and receives strong projections from somatosensory cortex11,12. Previous studies on prenatal alcohol exposure demonstrated altered sensory processing13, and abnormal sensory processing has been associated with altered executive function14. This sex difference in ethanol-induced connectivity changes might explain at least in part, the previously reported sex differences in complex problem solving strategies2.Conclusion
This study revealed reduced fronto-striatal connectivity after prenatal alcohol exposure in male rats but not in female rats, consistent with previously observed sex difference in behavior. Alcohol exposed female rats demonstrated alteration in somatosensory cortico-striatal interaction suggesting potential deficits in sensory processing. Our findings suggest the necessity for studying the effects of prenatal alcohol exposure with sex as a critical factor.Acknowledgements
This study was supported by grants R01 AA022413 & AA024980.References
1 Rasmussen, C. Executive functioning and working memory in fetal alcohol spectrum disorder. Alcohol Clin Exp Res 29, 1359-1367 (2005).
2 Waddell, J. & Mooney, S. M. Choline and Working Memory Training Improve Cognitive Deficits Caused by Prenatal Exposure to Ethanol. Nutrients 9, doi:10.3390/nu9101080 (2017).
3 Monchi, O., Petrides, M., Strafella, A. P., Worsley, K. J. & Doyon, J. Functional role of the basal ganglia in the planning and execution of actions. Ann Neurol 59, 257-264, doi:10.1002/ana.20742 (2006).
4 Bonelli, R. M. & Cummings, J. L. Frontal-subcortical circuitry and behavior. Dialogues Clin Neurosci 9, 141-151 (2007).
5 Aron, A. R. et al. Converging evidence for a fronto-basal-ganglia network for inhibitory control of action and cognition. J Neurosci 27, 11860-11864, doi:10.1523/JNEUROSCI.3644-07.2007 (2007).
6 Owen, A. M., Doyon, J., Petrides, M. & Evans, A. C. Planning and spatial working memory: a positron emission tomography study in humans. Eur J Neurosci 8, 353-364 (1996).
7 Rogers, R. D., Andrews, T. C., Grasby, P. M., Brooks, D. J. & Robbins, T. W. Contrasting cortical and subcortical activations produced by attentional-set shifting and reversal learning in humans. J Cogn Neurosci 12, 142-162 (2000).
8 Valdes-Hernandez, P. A. et al. An in vivo MRI Template Set for Morphometry, Tissue Segmentation, and fMRI Localization in Rats. Front Neuroinform 5, 26, doi:10.3389/fninf.2011.00026 (2011).
9 Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society Series B. 57 (1), 289–300, doi:10.2307/2346101 (1995).
10 Bennett, C. M., Wolford, G. L. & Miller, M. B. The principled control of false positives in neuroimaging. Soc Cogn Affect Neurosci 4, 417-422, doi:10.1093/scan/nsp053 (2009).
11 Alloway, K. D., Crist, J., Mutic, J. J. & Roy, S. A. Corticostriatal projections from rat barrel cortex have an anisotropic organization that correlates with vibrissal whisking behavior. J Neurosci 19, 10908-10922 (1999).
12 Pidoux, M., Mahon, S., Deniau, J. M. & Charpier, S. Integration and propagation of somatosensory responses in the corticostriatal pathway: an intracellular study in vivo. J Physiol 589, 263-281, doi:10.1113/jphysiol.2010.199646 (2011).
13 Schneider, M. L. et al. Timing of moderate level prenatal alcohol exposure influences gene expression of sensory processing behavior in rhesus monkeys. Front Integr Neurosci 3, 30, doi:10.3389/neuro.07.030.2009 (2009).
14 Adams, J. N., Feldman, H. M., Huffman, L. C. & Loe, I. M. Sensory processing in preterm preschoolers and its association with executive function. Early Hum Dev 91, 227-233, doi:10.1016/j.earlhumdev.2015.01.013 (2015).
Figures