4978
Anti-angiogenic treatment alters stiffness of glioblastoma in an orthotopic mouse model1Institute of Neuroradiology, University Medical Center Goettingen, Goettingen, Germany, 2Department of Radiology, Brigham and Women's Hospital, Boston, MA, United States, 3Harvard Medical School, Boston, MA, United States, 4Department of Neurosurgery, Brigham and Women's Hospital, Boston, MA, United States, 5Department of Biomedical Engineering, Boston University, Boston, MA, United States, 6Department of Radiological Imaging, Imaging Sciences & Biomedical Engineering Division, King's College London, London, United Kingdom
Synopsis
Glioblastoma (GBM) is the most common malignant brain tumor. As it is highly vascularized, anti-angiogenic treatment strategies have been tested. Such treatment hinders radiological tumor monitoring. Here, we probed the potential of magnetic resonance elastography (MRE) to evaluate GBM treated with anti-angiogenic therapy. GBM was orthotopically implanted in 10 nude mice, of which 5 were treated with B20 anti-VEGF antibody. MRI and MRE were performed repeatedly and brains were harvested for histology afterwards. Anti-angiogenic treatment slowed tumor growth, affected contrast-enhancement and slowed down tumor softening. The phase angle Y expressing the solid/liquid ratio appeared to indicate tumor progression.
Introduction
Glioblastoma (GBM) is the most common primary brain tumor. It is highly malignant and has a poor prognosis with a 5-year survival rate of less than 10%1. Pathological hallmarks of GBM include highly invasive growth, regional heterogeneity, necrosis and microvascular proliferation2. Hence, blocking of angiogenesis with monoclonal antibodies in addition to standard therapy consisting of resection followed by radiotherapy and temozolomide was tested in patients with GBM3. With this, patients had prolonged progression free survival and maintained baseline quality of life longer, which was ascribed to normalization of the vasculature caused by anti-angiogenic treatment4. This however complicates radiological tumor monitoring and standard MRI techniques have shortcomings in providing objective measures of tumor progression5. Hence, we investigated whether magnetic resonance elastography (MRE) could improve evaluation of GBM under anti-angiogenic treatment. The aim was to probe the potential of MRE to differentiate between treated and untreated tumors and to test, whether MRE can distinguish between treatment response and tumor progression.Methods
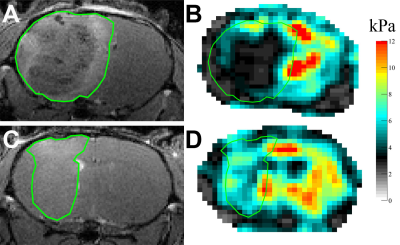
All experiments were performed in accordance with the local institutional animal care and use committee. G9 tumor cells established from a patient tissue sample under IRB approval were surgically implanted into the right striatum of 10 athymic nude mice. Five animals received i.p. injections of 10 mg B20 anti-VEGF-antibody 4, 6 and 8 days after tumor implantation. The other 5 animals remained untreated and served as controls. MRI comprising T2w and contrast-enhanced T1w sequences as well as MRE imaging was performed 6, 8 and 10 days after implantation on a 7T small animal scanner. Then, animals were sacrificed for histological analyses. Regions of interest (ROIs) covering the whole tumor, the contrast-enhancing tumor parts, a 600 μm thick peripheral rim around the tumor and normal appearing brain tissue (NABT) in the contralateral hemisphere were defined on T1w images. Then, tumor volume as well was relative T1w signal intensities of tumor and NABT were established. The same ROIs were copied to MRE maps and mean and standard deviation of the viscoelastic modulus |G*| and the phase angle Y were calculated (|G*| = √Gd2 + Gl2; Y =2/π atan(Gl/Gd); Gd = elastic shear modulus, Gl = loss shear modulus). |G*| informs about tissue rigidity, while Y indicates whether elastic (Y ≈ 0) or viscous (Y ≈ 1) properties prevail in the tissue. All parameters were compared between treated and untreated animals using one- or two-way ANOVA with Tukey’s post-test as appropriate.Results
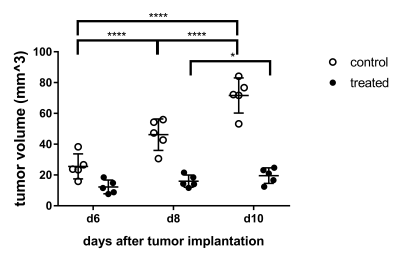
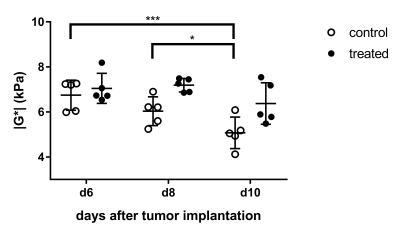
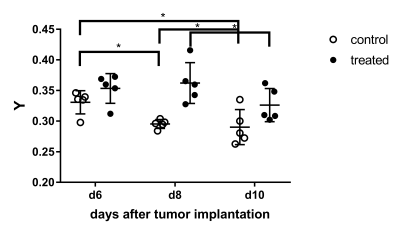
Treated and untreated tumors showed significant differences in multiple parameters. Tumor volume largely increased in untreated controls, while there was only a small increase under treatment with B20 (p < 0.01; Fig. 1, 2). Similarly, there was a significant increase in contrast-enhancement in untreated mice, whereas the amount of contrast-enhancing tumor tissue was stable in treated animals (p < 0.01). Additionally, relative T1w signal intensity of the whole tumor as well as the contrast-enhancing components were analyzed. Again, significant differences between untreated and treated animals could be seen (p = 0.01 and p = 0.03). The analysis of MRE parameters revealed that tumors in untreated animals were significantly softer (Fig. 1) and had significantly lower values of Y indicating prevailing elastic properties compared to tumors under anti-angiogenic treatment with B20 (p = 0.02 for |G*| and p < 0.01 for Y; Figs. 3, 4). While the viscoelastic modulus progressively decreased in untreated controls, it did not change in treated animals. Y similarly decreased in controls. In treated animals, a drop in Y was observed at day 10. The analysis of the peripheral rim showed neither for |G*|, nor for Y significant differences between treated and untreated tumors. Histological analyses showed that treated tumors were more invasive than untreated tumors. Untreated tumors were more necrotic. Additionally, vessel architecture differed between treated and untreated tumors.Discussion
Our findings suggest that B20 treatment slowed down tumor progression and led to a normalization of the blood brain barrier. These findings are in line with the literature as others described GBM to be smaller and less contrast-enhancing when treated with anti-angiogenic agents6,7. Moreover, B20 treatment decelerated tumor softening, which has been shown with progression8. Interestingly, Y decreased significantly 10 days after implantation in treated animals, while the viscoelastic modulus remained stable and tumor volume only slightly increased. Y appeared to be influenced by vessel architecture and tissue integrity and might provide early information on tumor progression. The invasiveness of treated tumors was not reflected by differences of MRE parameters in the peripheral rim. This is most likely due to limited spatial resolution of MRE.Conclusion
Anti-angiogenic treatment decelerated tumor growth and softening. The MRE parameter Y could potentially provide early information on GBM progression.Acknowledgements
The authors thank Hong Zhang, Ph.D. for valuable technical support and Charles RG Guttmann, M.D. for excellent discussions. We acknowledge grant support from NIH R21 EB020757, from European Commission Horizon 2020 proposal 668039 and from Boston University College of Engineering and the Brigham and Women’s Hospital Department of Radiology. K.S. received funding from the German Research Foundation (DFG, SCHR 1542/1-1).References
1. Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987-996. doi:10.1056/NEJMoa043330.
2. Brandes AA, Tosoni A, Franceschi E, et al. Glioblastoma in adults. Crit Rev Oncol Hematol. 2008;67(2):139-152. doi:10.1016/j.critrevonc.2008.02.005.
3. Chinot OL, Wick W, Mason W, et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):709-722. doi:10.1056/NEJMoa1308345.
4. Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307(5706):58-62. doi:10.1126/science.1104819.
5. Wen PY, Macdonald DR, Reardon DA, et al. Updated Response Assessment Criteria for High-Grade Gliomas: Response Assessment in Neuro-Oncology Working Group. J Clin Oncol. 2010;28(11):1963-1972. doi:10.1200/JCO.2009.26.3541.
6. Keunen O, Johansson M, Oudin A, et al. Anti-VEGF treatment reduces blood supply and increases tumor cell invasion in glioblastoma. Proc Natl Acad Sci. 2011;108(9):3749-3754. doi:10.1073/pnas.1014480108.
7. Jalali S, Chung C, Foltz W, et al. MRI biomarkers identify the differential response of glioblastoma multiforme to anti-angiogenic therapy. Neuro-Oncol. 2014;16(6):868. doi:10.1093/neuonc/nou040.
8. Schregel K, Nazari N, Nowicki MO, et al. Characterization of glioblastoma in an orthotopic mouse model with Magnetic Resonance Elastography. NMR Biomed. doi:10.1002/nbm.3840 in press
Figures