4977
Longitudinal evaluation of lesion pathology in a novel murine model for Multiple Sclerosis1Department of Radiology and Biomedical Imaging, University of California San Francisco, San Francisco, CA, United States, 2Department of Physical Therapy and Rehabilitation Science, University of California San Francisco, San Francisco, CA, United States, 3Department of Neurology, University of California San Francisco, San Francisco, CA, United States
Synopsis
We used conventional MRI to longitudinally assess lesion pathology in a combined cuprizone and experimental autoimmune encephalomyelitis (CPZ/EAE) model. The novelty of this model lies in the recruitment of cells from the innate and adaptive immune system into brain lesions. We used T2-weighted imaging to monitor white matter lesions. Interestingly, we showed a transient change in T2 contrast at the onset of clinical symptoms in the CPZ/EAE group. Using gadolinium-enhanced MRI, we showed transient opening of the blood-brain-barrier prior and/or following clinical symptoms. Altogether, these findings are of relevance to understand the dynamics of lesion formation in a novel MS model.
Introduction
Multiple Sclerosis (MS) is a neuroinflammatory disorder of the central nervous system characterized by myelin loss, axonal damage, recruitment and activation of resident and peripheral immune cells, with the vast majority of lesions present in the brain. Existing animal models for MS present limitations in terms of location of lesions (spinal cord) or physiopathology (absence of monocytes and/or T-cell infiltration). Recent studies1,2 have shown that the combination of the two main preclinical models for MS, the experimental autoimmune encephalomyelitis3 (EAE) and the cuprizone4 (CPZ) models, leads to the formation of brain lesions presenting a strong infiltration of monocytes and activated T-cells. In this study, we longitudinally followed the lesions formation of this newly introduced combined CPZ/EAE model. Using conventional MRI, we were able to monitor lesion expansion, partial recovery and leaky blood brain barrier (BBB).Methods
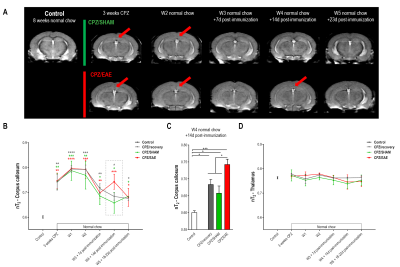
Animals and experimental setup: Adult C57/BL6J mice were separated in four groups: 1) Control (n=3), 2) CPZ/recovery (n=6), 3) CPZ/SHAM (n=6) and 4) CPZ/EAE (n=6). Control received a normal chow while groups 2, 3 and 4 received a CPZ diet (0.25%) for three weeks to induce brain demyelination and neuroinflammation, and were returned to a normal chow for five additional weeks. Groups 3 and 4 were SHAM-immunized or MOG35-55-immunized, respectively, as previously described1 (Fig.1A).
EAE scoring: Scoring of disease severity was performed as followed: 0) normal, 1) decreased tail tone, 2) hind limb weakness, 3) hind limb paralysis, 4) forelimbs weakness/paraplegia, 5) limbs paralysis.
MR acquisitions: MR acquisitions were performed on a 14.1T MR scanner. Mice (n≥3 per group) were imaged after three weeks of CPZ diet and every following week (Fig.1A). T2-weighted images were acquired using: TE/TR=12/2000ms, 20 slices, thickness=0.5mm, NA=8, matrix=256x256, FOV=30x30mm². In order to evaluate BBB integrity T1-weighted images were acquired prior and five minutes post-gadolinium-DTPA injection (1 mmol/kg) in the tail vein using: TE/TR=2/120ms, flip angle=40º, 10 slices, thickness=0.8mm, gap=0.2mm, NA=10, matrix=256x256, FOV=20x20mm²)
Immunofluorescence: Immunofluorescence analyses were performed at the end of the experiment for myelin basic protein (MBP), microglia/macrophages (Iba-1), CD3 T-cells and nuclei (Hoechst).
Data analyses: The corpus callosum (CC) and thalamus were manually delineated on T2-weighted images and the average T2 signals were normalized (nT2) to T2 from the cerebrospinal fluid. Statistical significance was evaluated using a One-Way ANOVA with post-hoc Tukey (*p<0.05, **p<0.01, ***p<0.001, ****p<0.0001).
Results
In this study, only mice from the CPZ/EAE group developed EAE symptoms, starting day 9 post-immunization, with progressive worsening of the symptoms (Fig.1B).
Longitudinal analyses of T2-weighted images showed:
- Increased nT2 values from the CC at 3 weeks of CPZ diet compared to control (p≤0.0053) indicating demyelination and neuroinflammation (Fig.2A-B).
- nT2 values remained elevated for two weeks thereafter, demonstrating lesion maintenance despite withdrawal of the toxic compound.
- Decreased nT2 values after 3 weeks of normal chow, indicative of remyelination and/or decreased inflammation.
- CPZ/recovery, CPZ/SHAM and CPZ/EAE groups showed similar nT2 values at every time point, except 14d post-immunization when nT2 values were significantly increased in CPZ/EAE (p<0.0355) (Fig.2B-C).
- nT2 values from the thalamus remained unchanged compared to control (Fig.2D, p=0.9562).
Immunofluorescence analyses performed after 5 weeks of normal chow showed an important presence of macrophages and CD3 T-cells in the CC of CPZ/EAE mice compared to other groups (Fig.3A-C, p<0.0001 and p≤0.0017, respectively), while no differences in myelin could be observed (Fig3.D, p=0.3347).
Gadolinium-enhanced MRI revealed a leaky BBB (Fig.4A) in the vicinity of the major blood vessels and beneath the hippocampus only in a subset of the CPZ/EAE mice (n=2 mice at 7d and 14d, n=1 mouse at 23d post-immunization). BBB opening was transient and was not associated with EAE-symptoms onset nor severity.
Immunofluorescence analyses indicated that only the CPZ/EAE group showed inflammatory lesions with CD3 T-cell recruitment beneath the hippocampal formation, corresponding to the location of gadolinium enhancement on MR images (Fig.4B). Additionally, immunofluorescence analyses showed smaller inflammatory lesions in the CPZ/EAE group within the external capsule and the thalamus.
Discussion
We showed that conventional MR methods such as T2-weighted and gadolinium-enhanced imaging were able to monitor lesion expansion and recovery in the combined CPZ/EAE model, providing non-invasive tools to study lesion pathology dynamics in vivo.
At the onset of EAE symptoms, nT2 signals from the CC transiently increased, which may be linked to fluid accumulation and alteration of aquaporin-4 water-channels2. We noted that BBB transiently opened only in a subset of mice from the CPZ/EAE group. As we imaged mice weekly, it is likely that BBB leakage may have occurred between imaging time points.
Future studies will aim at investigating the metabolic signature of the combined CPZ/EAE model5.
Acknowledgements
This work was supported by research grants from the NIH R01NS102156; Cal-BRAIN 349087; NMSS research grant RG-1701-26630; Hilton Foundation – Marilyn Hilton Award for Innovation in MS Research #17319; Dana Foundation: The David Mahoney Neuroimaging program, and fellowship from the NMSS FG-1507-05297.References
1 Ruther, B. J. et al. Combination of cuprizone and experimental autoimmune encephalomyelitis to study inflammatory brain lesion formation and progression. Glia 65, 1900-1913, doi:10.1002/glia.23202 (2017).
2 Scheld, M. et al. Neurodegeneration Triggers Peripheral Immune Cell Recruitment into the Forebrain. J Neurosci 36, 1410-1415, doi:10.1523/JNEUROSCI.2456-15.2016 (2016).
3 Didonna, A. Preclinical Models of Multiple Sclerosis: Advantages and Limitations Towards Better Therapies. Curr Med Chem 23, 1442-1459 (2016).
4 Praet, J., Guglielmetti, C., Berneman, Z., Van der Linden, A. & Ponsaerts, P. Cellular and molecular neuropathology of the cuprizone mouse model: clinical relevance for multiple sclerosis. Neurosci Biobehav Rev 47, 485-505, doi:10.1016/j.neubiorev.2014.10.004 (2014).
5 Guglielmetti, C. et al. Hyperpolarized 13C MR metabolic imaging can detect neuroinflammation in vivo in a multiple sclerosis murine model. Proc Natl Acad Sci U S A 114, E6982-E6991, doi:10.1073/pnas.1613345114 (2017).
Figures