4972
Lactate, the new hope against brain damages in neonatal hypoxia-ischemia1Centre de Résonance Magnétique des Systèmes Biologiques - UMR 5536 - CNRS, BORDEAUX, France
Synopsis
Hypoxia-ischemia remains a major perinatal mortality and chronic disability cause in newborns. Hypothermia is the only clinically therapeutic approach but new therapy must be developed. Our aim was to study the neuroprotective role of lactate in rat neonatal hypoxic-ischemic model and to determine the best pattern to counteract brain damages. Interestingly, lactate was much more neuroprotective when administered after hypoxia (curative) than before (preventive). Single versus 48h injection of lactate were compared. Lactate shows significant and important neuroprotection (ADC, FA, immunohistochemistry, behavioral studies).
Introduction
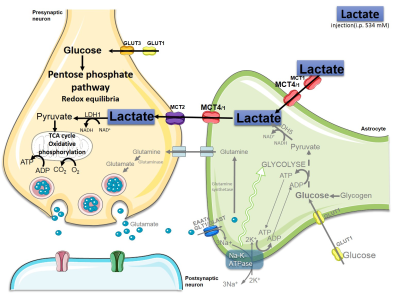
Hypoxic ischemic (HI) encephalopathy remains a major cause of perinatal mortality and chronic disability in newborns worldwide (1-6 for 1,000 births) [1, 2]. The only current clinical treatment is hypothermia which is efficient for less than 60% of babies. Therefore, new therapeutic approaches must be developed. If lactate was previously considered as a waste product in the past, it is now more and more admitted to be a supplementary fuel for neurons via the astrocyte-neuron lactate shuttle [3]. Moreover, lactate was recently proposed to be an important signaling molecule in the brain. To our knowledge, no exploration of neuroprotective role of lactate was done in neonatal HI context. Our aim was to investigate the neuroprotective effect of lactate in a rat neonatal model of hypoxia-ischemia (Vannucci-Rice model in P7 pups). We followed in vivo by diffusion MRI at 4.7T brain damages. Immunohistochemistry as well as motor and behavioral studies were carried out in parallel to evaluate the neuroprotective effect of lactate.Methods
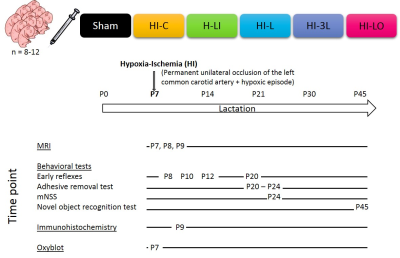
Neonates Wistar rats (P7) underwent a HI lesion (left carotid artery ligation + hypoxia: 8%O2, 92%N2, 2h) [4]. Experimental design is presented in Figure1.Results
Preventive or curative?
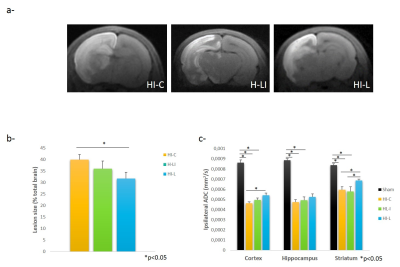
Three hours after HI, HI-L group showed a significant reducing in damaged area, compared to HI-C group (31.64±2.82% Vs 39.78±2.80% of the total brain, respectively; P<0.05)(Figure2a-b). ADC significantly decreased for all HI groups compared to Sham group (Figure2c). HI-L group showed a significant higher ADC in cortex and striatum (compared to HI-C and HL-I).
Metabolic or signalling effect?
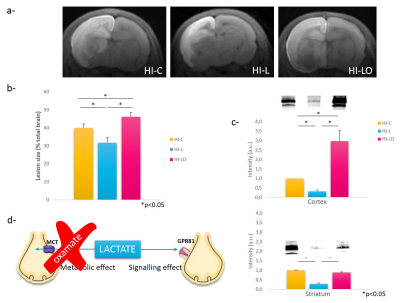
Co-injection of oxamate significantly increased brain damages (lesion sizes: 39.77+2.80%, 31.64+2.83%, and 46.03+2.40% for HI-C, HI-L and HI-LO groups, respectively) (Figure 3 a-b). HI-L group presented a significant decrease in ROS production compared to HI-C group in the cortex (-68%) and in the striatum (-72%) (Figure 3c). HI-LO group presented a significant increase in ROS production in the cortex compared to HI-C group (+200%).
Acute or long-term effect?
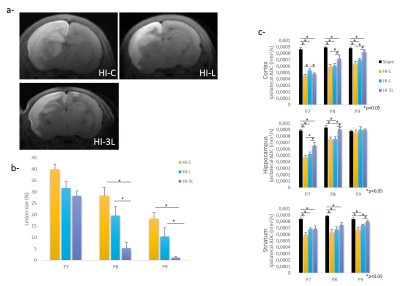
From 24h post-HI, HI-3L group had significantly the lowest lesion volume when compared to the other groups (1.15+0.52% Vs 18.29+2.71% and 10.38+3.85% for HI-3L group Vs HI-C and HI-L groups, respectively, at P9) (Figure4a-b). At P9, HI-3L group presented the best recovery of ADC values in cortex and striatum (Figure4c).
Immunohistological characterization.
Abundance of CASP3 was higher in HI-C group compared to Sham group and lactate injection led to a down-regulation of CASP3. AQP4 seemed to be down-regulated in HI-C group compared to Sham and HI-L groups. At P9, the abundance of MCT1 did not appeared to be affected by either HI or lactate injection while MCT4 was down-regulated by HI and up-regulated by lactate injection.
Neurological reflexes and sensorimotor repercussion.
HI-C group presented significantly poor performances in the righting reflex while no difference was measured between Sham and HI-3L. Likewise, Sham and HI-3L groups presented better performances in the adhesive removal test than for HI-C group, at P23.
Long-term memory.
HI induced a significant decrease in the discrimination in the novel object recognition test compared to Sham group that was reverted in HI-3L group (0.77+0.04; 0.62+0.08; 0.81+0.03, for Sham, HI-C and HI-L groups, respectively).
Discussion
The neuroprotective effect of lactate was the most effective when administered after hypoxia, highlighting its clinical potential. Lactate neuroprotection can act through two different pathways (see Figure 3d). To distinguish between the metabolic and signalling route, we used oxamate to block the LDH and showed that neuroprotection was abolished, indicating that the metabolism of lactate is necessary to get neuroprotection. Interestingly, brain lesions in HI-LO were even bigger than in control pups, suggesting that lactate, produced endogenously by the brain during hypoxia, was already neuroprotective. This neuroprotection was correlated with increased AQP4 and MCT4 staining and recovery of cognitive functions.Conclusion
To our knowledge, this is the first in vivo evidence of a neuroprotective role of lactate in the neonatal hypoxia-ischemia context. Our results strengthened the idea that lactate should not be considered anymore as a waste product but rather as a crucial molecule for the brain and its important roles: metabolic, as a substrate for neurons, and neuroprotective.Acknowledgements
No acknowledgement found.References
1. Kurinczuk, J.J., M. White-Koning, and N. Badawi, Epidemiology of neonatal encephalopathy and hypoxic-ischaemic encephalopathy. Early Hum Dev, 2010. 86(6): p. 329-38.
2. Davidson, J.O., et al., Therapeutic Hypothermia for Neonatal Hypoxic-Ischemic Encephalopathy - Where to from Here? Front Neurol, 2015. 6: p. 198.
3. Pellerin, L. and P.J. Magistretti, Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci U S A, 1994. 91(22): p. 10625-9.
4. Rice, J.E., 3rd, R.C. Vannucci, and J.B. Brierley, The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann Neurol, 1981. 9(2): p. 131-41.
Figures