4971
Comparison of white matter tracts between macaque and human brain1Department of Radiology, Children hospital of Philadelphia, Philadelphia, PA, United States, 2Department of Radiology, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, United States, 3School of Psychological and Cognitive Sciences, Peking University, Beijing, China, 4Peking-Tsinghua Center for Life Science, Peking University, Beijing, China
Synopsis
White matter as the substrate of connectivity plays a critical role in brain evolution. However, the comprehensive tract-level comparison in morphology and microstructure between all common macaque and human white matter tracts has not been delineated. We aimed to qualitatively and quantitatively compare the tract-level microstructure and 3D pathways of corresponding white matter tracts between macaque and human brain using DTI measurements and tractography. Macaque white matter tract skeleton was obtained and comparison was conducted in five categorized tract groups. The differences are most prominent in association tracts with more extensive pathways and higher microstructural integrity in human brain association tracts.
Purpose
White matter as the substrate of connectivity plays a critical role in brain evolution. Morphological differences of specific white matter tracts such as the arcuate fasciculus between macaque and human brain [1] have been revealed based on DTI tractography. However, the comprehensive tract-level comparison in morphology and microstructure of all common white matter tracts between rhesus macaque (Macaca mulatta) and human brain have not been reported. A comprehensive characterization of white matter anatomy of macaque brain with DTI can not only offer insights into brain evolution, but also provide neuroanatomical basis for neuroscientific studies using macaque model [2]. We hypothesized that, in parallel to cerebral cortical evolution, significant and selective white matter evolution occurs from macaque to human brain. In this study, we aimed to qualitatively and quantitatively compare the tract-level microstructure and 3D pathways of corresponding white matter tracts between macaque and human brain using DTI-derived metric measurements and DTI-based tractography. Macaque white matter tract skeleton was obtained and comparison was conducted in all five categorized functional tract groups.Methods
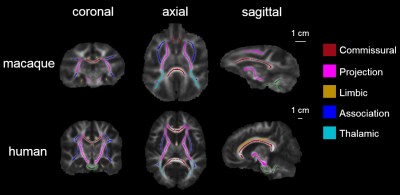
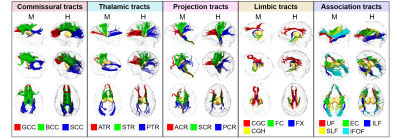
Subjects and data acquisition: Ten young adult macaques (age: 5.3±2.8 years; 4 female) and fifteen healthy young adults (age: 20.5±3.6 years; 7 female) human subjects were recruited. In vivo diffusion MRI (dMRI) was acquired from a 3T Philips Achieva system using a single-shot EPI sequence. The dMRI imaging parameters for macaques were: TE = 71 ms, TR = 5410 ms, in-plane field of view = 150 x 150 mm2 (imaging resolution of 1.5mm), slice thickness = 1.5 mm, slice number = 70, 30 independent diffusion encoding directions, b-value = 1000 sec/mm2. The dMRI imaging parameters for human were: TE = 97 ms, TR = 7780 ms, in-plane field of view = 224 x 224 mm2 (imaging resolution of 2 mm), slice thickness = 2.2 mm, slice number = 65, 30 independent diffusion encoding directions, b-value = 1000 sec/mm2. DTI tractography in five tract groups: Streamline tractography [3] was used for DTI fiber tracking of all in vivo macaque and human brain DTI data. The tractography protocol for tracing five categories of white matter tracts in human brain [4] was also used to trace tracts in the macaque brains. Microstructural maturation of white matter tracts and tract groups: Digital white matter atlas were used to parcellate white matter tracts and tract groups for macaque [5] and human [6], respectively. Nonlinear registration, skeletonization and projection steps from FSL TBSS (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/TBSS) were used to obtain the white matter skeleton and map the atlas labels to the white matter skeleton in the averaged fractional anisotropy (FA) maps, as shown in Fig 1. The single-subject template used for nonlinear registration process in TBSS was identical to the template used for establishing the white matter atlas. Each skeleton voxel was categorized in one of the major tracts and one of the five tract groups: commissural, projection, thalamic, limbic and association tract group [4]. The FA threshold at the white matter skeleton is 0.2 to keep only deep white matter with continuous skeleton voxels (Fig 1).Results
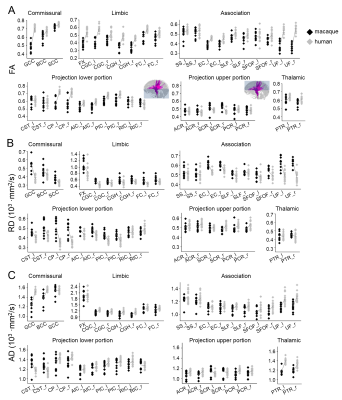
The three-dimensional pathways of common macaque and human white matter tracts: As shown in Fig 2, the three-dimensional pathways of commissural, thalamic, projection and limbic tracts of macaque brain are similar to those of human brain. Significant differences between macaque and human brain are apparent in association tracts. Especially the arcuate fasciculus, related to language and other functions unique to humans, could not be reliably traced in macaque brain. Differentiated enhancement of tract-level microstructural integrity in five tract groups between macaque and human: Compared to human white matter tracts, significantly lower FA, lower axial diffusivity (AD) and higher radial diffusivity (RD) measurements in limbic tracts, lower portion of projection tracts and tracts connecting to prefrontal brain (uncinate fasciculus and genu of corpus callosum) were found in macaque brain. The reverse pattern was shown in upper portion projection tracts, especially right superior corona radiation, where higher FA and higher AD and lower RD was found in macaque brain.Discussion and conclusion
Based on comprehensive tract-level microstructural measurements at macaque and human brain white matter skeletons, the results revealed enhanced myelination in selective prefrontal white matter tracts from macaque to human. This study has provided unique and comprehensive insights on microstructural changes of common white matter tracts and hence extended our understanding of anatomical evolution from macaque to human.Acknowledgements
This study is funded by NIH MH092535, MH092535-S1 and HD086984.References
[1] Rilling et al. (2008) Nature Neuroscience 11:4.
[2] Steinmetz et al. (2000) Nature 404: 187.
[3] Mori et al. (1999) Annals of Neurology 45: 265.
[4] Wakana et al. (2004) Radiology 230: 77.
[5] Feng et al. (2017) Structure and Function : 1-17.
[6] Mori et al. (2008) Neuroimage 40: 570.
Figures